当前位置:
X-MOL 学术
›
J. Am. Coll. Cardiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quality Assurance for Carotid Stenting in the CREST-2 Registry
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.jacc.2019.10.032 Brajesh K Lal 1 , Gary S Roubin 2 , Kenneth Rosenfield 3 , Donald Heck 4 , Michael Jones 5 , Brian Jankowitz 6 , Tudor Jovin 7 , Seemant Chaturvedi 8 , Guilherme Dabus 9 , Christopher J White 10 , William Gray 11 , Jon Matsumura 12 , Barry T Katzen 13 , L Nelson Hopkins 14 , Minerva Mayorga-Carlin 1 , John D Sorkin 15 , George Howard 16 , James F Meschia 17 , Thomas G Brott 17
Journal of the American College of Cardiology ( IF 24.0 ) Pub Date : 2019-12-01 , DOI: 10.1016/j.jacc.2019.10.032 Brajesh K Lal 1 , Gary S Roubin 2 , Kenneth Rosenfield 3 , Donald Heck 4 , Michael Jones 5 , Brian Jankowitz 6 , Tudor Jovin 7 , Seemant Chaturvedi 8 , Guilherme Dabus 9 , Christopher J White 10 , William Gray 11 , Jon Matsumura 12 , Barry T Katzen 13 , L Nelson Hopkins 14 , Minerva Mayorga-Carlin 1 , John D Sorkin 15 , George Howard 16 , James F Meschia 17 , Thomas G Brott 17
Affiliation
|
BACKGROUND
The CREST-2 Registry (C2R) was approved by National Institute of Neurological Disorders and Stroke-National Institutes of Health in September 2014 with Centers for Medicare & Medicaid Services, U.S. Food and Drug Administration, and industry collaboration to enroll patients undergoing CAS. The registry credentials interventionists and promotes optimal patient selection, procedural-technique, and outcomes. OBJECTIVES
This study reports periprocedural outcomes in a cohort of carotid artery stenting (CAS) performed for asymptomatic and symptomatic carotid stenosis. METHODS
Asymptomatic patients with ≥70% and symptomatic patients with ≥50% carotid stenosis, ≤80 years of age, and at standard or high risk for carotid endarterectomy are eligible for enrollment. Interventionists are credentialed by a multispecialty committee that reviews experience, lesion selection, technique, and outcomes. The primary endpoint was a composite of stroke and death (S/D) in the 30-day periprocedural period. Myocardial infarction and access-site complications were assessed as secondary outcomes. RESULTS
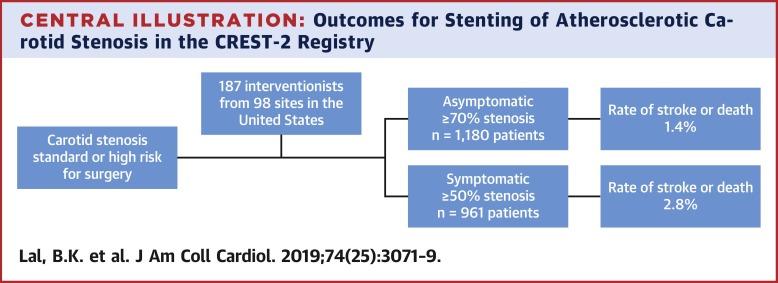
As of December 2018, 187 interventionists from 98 sites in the United States performed 2,219 CAS procedures in 2,141 patients with primary atherosclerosis (78 were bilateral). The mean age of the cohort was 68 years, 65% were male, and 92% were white; 1,180 (55%) were for asymptomatic disease, and 961 (45%) were for symptomatic disease. All U.S. Food and Drug Administration-approved stents and embolic protection devices were represented. The 30-day rate of S/D was 1.4% for asymptomatic, 2.8% for symptomatic, and 2.0% for all patients. CONCLUSIONS
C2R is the first national registry for CAS cosponsored by federal and industry partners. CAS was performed by experienced operators using appropriate patient selection and optimal technique. In that setting, a broad group of interventionists achieved very low periprocedural S/D rates for asymptomatic and symptomatic patients.
中文翻译:

CREST-2 登记处颈动脉支架置入术的质量保证
背景 CREST-2 注册 (C2R) 于 2014 年 9 月获得美国国立神经疾病和中风研究所 - 美国国立卫生研究院与医疗保险和医疗补助服务中心、美国食品和药物管理局以及行业合作的批准,用于招募接受 CAS 的患者。登记处认证干预者并促进最佳患者选择、程序技术和结果。目的 本研究报告了一组颈动脉支架置入术 (CAS) 治疗无症状和有症状颈动脉狭窄的围手术期结果。方法 颈动脉狭窄≥70%的无症状患者和颈动脉狭窄≥50%的有症状患者,年龄≤80岁,颈动脉内膜切除术标准或高危患者均符合入选条件。介入医师由一个多专业委员会认证,该委员会审查经验、病变选择、技术和结果。主要终点是围手术期 30 天的复合卒中和死亡 (S/D)。心肌梗塞和通路部位并发症被评估为次要结果。结果 截至 2018 年 12 月,来自美国 98 个地点的 187 名介入医师对 2,141 名原发性动脉粥样硬化患者(78 名为双侧)进行了 2,219 次 CAS 手术。该队列的平均年龄为 68 岁,65% 为男性,92% 为白人;1,180 人(55%)为无症状疾病,961 人(45%)为有症状疾病。代表了所有美国食品和药物管理局批准的支架和栓塞保护装置。无症状患者的 30 天 S/D 率为 1.4%,有症状患者为 2.8%,2. 所有患者均为 0%。结论 C2R 是第一个由联邦和行业合作伙伴共同发起的 CAS 国家注册。CAS 由经验丰富的操作员使用适当的患者选择和最佳技术进行。在这种情况下,广泛的干预专家组在无症状和有症状患者中实现了非常低的围手术期 S/D 率。
更新日期:2019-12-01
中文翻译:

CREST-2 登记处颈动脉支架置入术的质量保证
背景 CREST-2 注册 (C2R) 于 2014 年 9 月获得美国国立神经疾病和中风研究所 - 美国国立卫生研究院与医疗保险和医疗补助服务中心、美国食品和药物管理局以及行业合作的批准,用于招募接受 CAS 的患者。登记处认证干预者并促进最佳患者选择、程序技术和结果。目的 本研究报告了一组颈动脉支架置入术 (CAS) 治疗无症状和有症状颈动脉狭窄的围手术期结果。方法 颈动脉狭窄≥70%的无症状患者和颈动脉狭窄≥50%的有症状患者,年龄≤80岁,颈动脉内膜切除术标准或高危患者均符合入选条件。介入医师由一个多专业委员会认证,该委员会审查经验、病变选择、技术和结果。主要终点是围手术期 30 天的复合卒中和死亡 (S/D)。心肌梗塞和通路部位并发症被评估为次要结果。结果 截至 2018 年 12 月,来自美国 98 个地点的 187 名介入医师对 2,141 名原发性动脉粥样硬化患者(78 名为双侧)进行了 2,219 次 CAS 手术。该队列的平均年龄为 68 岁,65% 为男性,92% 为白人;1,180 人(55%)为无症状疾病,961 人(45%)为有症状疾病。代表了所有美国食品和药物管理局批准的支架和栓塞保护装置。无症状患者的 30 天 S/D 率为 1.4%,有症状患者为 2.8%,2. 所有患者均为 0%。结论 C2R 是第一个由联邦和行业合作伙伴共同发起的 CAS 国家注册。CAS 由经验丰富的操作员使用适当的患者选择和最佳技术进行。在这种情况下,广泛的干预专家组在无症状和有症状患者中实现了非常低的围手术期 S/D 率。


























 京公网安备 11010802027423号
京公网安备 11010802027423号