当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Mechanochemical Asymmetric Cross‐Dehydrogenative Coupling Reaction: Liquid‐Assisted Grinding Enables Reaction Acceleration and Enantioselectivity Control
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1002/adsc.201901363 Jingbo Yu 1 , Ping Ying 2 , Hao Wang 1 , Keyu Xiang 1 , Weike Su 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-13 , DOI: 10.1002/adsc.201901363 Jingbo Yu 1 , Ping Ying 2 , Hao Wang 1 , Keyu Xiang 1 , Weike Su 1
Affiliation
|
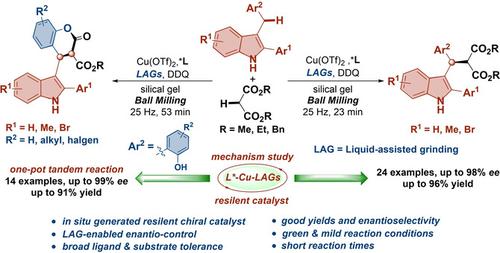
We report here a mechanochemical protocol for asymmetric cross‐dehydrogenative coupling (CDC) reaction of unreactive 3‐arylmethyl indoles with 1,3‐dicarbonyl by liquid‐assisted grinding. Small drops of liquid additive n‐butyl acetate (n‐BuOAc) are key to achieve high yield and enantioselectivity in this transformation. The catalyst can be lowered to 5 mol% and reused for three times. Pharmaceutical useful chiral dihydrocoumarins are further achieved through one‐pot tandem asymmetric CDC and cyclization with excellent enantioselectivities. Experimental and computational studies on the reaction mechanism suggest that n‐BuOAc is participated to the catalytic cycle, forming a chiral metal species during the reaction process and thereby revealed the origin of stereoselectivity.
中文翻译:

机械化学不对称交叉脱氢偶联反应:液体辅助研磨可促进反应加速和对映选择性控制
我们在这里报告了一种化学化学方法,用于通过液体辅助研磨将未反应的3-芳基甲基吲哚与1,3-二羰基进行不对称的交叉脱氢偶联(CDC)反应。液体添加剂乙酸正丁酯(n- BuOAc)的小滴滴滴是实现此转化过程中高收率和对映选择性的关键。催化剂可降低至5mol%并重复使用三次。通过一锅串联不对称CDC和具有出色对映选择性的环化,可进一步获得可药用的手性二氢香豆素。实验和反应机理的计算研究表明,ñ‐BuOAc参与催化循环,在反应过程中形成手性金属,从而揭示了立体选择性的起源。
更新日期:2020-01-13
中文翻译:

机械化学不对称交叉脱氢偶联反应:液体辅助研磨可促进反应加速和对映选择性控制
我们在这里报告了一种化学化学方法,用于通过液体辅助研磨将未反应的3-芳基甲基吲哚与1,3-二羰基进行不对称的交叉脱氢偶联(CDC)反应。液体添加剂乙酸正丁酯(n- BuOAc)的小滴滴滴是实现此转化过程中高收率和对映选择性的关键。催化剂可降低至5mol%并重复使用三次。通过一锅串联不对称CDC和具有出色对映选择性的环化,可进一步获得可药用的手性二氢香豆素。实验和反应机理的计算研究表明,ñ‐BuOAc参与催化循环,在反应过程中形成手性金属,从而揭示了立体选择性的起源。


























 京公网安备 11010802027423号
京公网安备 11010802027423号