当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Herpesviruses induce aggregation and selective autophagy of host signalling proteins NEMO and RIPK1 as an immune-evasion mechanism.
Nature Microbiology ( IF 28.3 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41564-019-0624-1 Elena Muscolino 1 , Rebekka Schmitz 1 , Stefan Loroch 2 , Enrico Caragliano 1 , Carola Schneider 1 , Matteo Rizzato 1 , Young-Hyun Kim 3, 4 , Eva Krause 1 , Vanda Juranić Lisnić 5 , Albert Sickmann 2 , Rudolph Reimer 1 , Eleonore Ostermann 1 , Wolfram Brune 1
Nature Microbiology ( IF 28.3 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41564-019-0624-1 Elena Muscolino 1 , Rebekka Schmitz 1 , Stefan Loroch 2 , Enrico Caragliano 1 , Carola Schneider 1 , Matteo Rizzato 1 , Young-Hyun Kim 3, 4 , Eva Krause 1 , Vanda Juranić Lisnić 5 , Albert Sickmann 2 , Rudolph Reimer 1 , Eleonore Ostermann 1 , Wolfram Brune 1
Affiliation
|
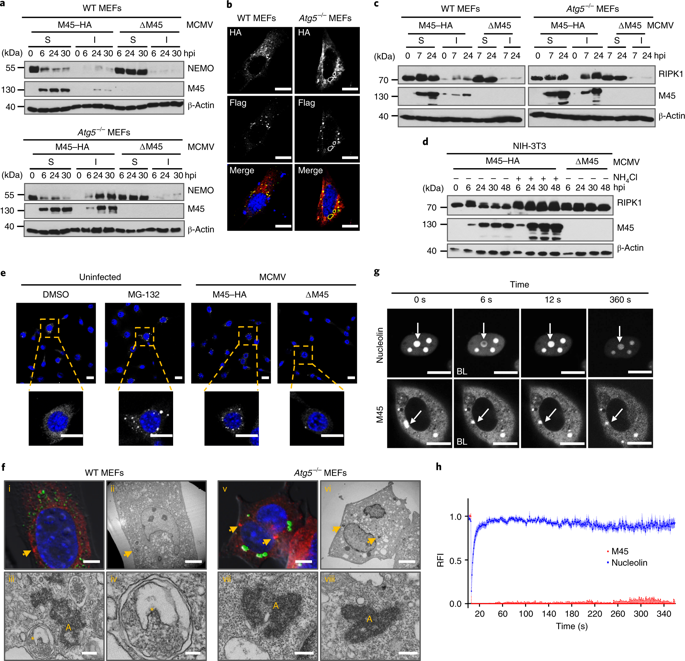
Viruses manipulate cellular signalling by inducing the degradation of crucial signal transducers, usually via the ubiquitin-proteasome pathway. Here, we show that the murine cytomegalovirus (Murid herpesvirus 1) M45 protein induces the degradation of two cellular signalling proteins, the nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) essential modulator (NEMO) and the receptor-interacting protein kinase 1 (RIPK1), via a different mechanism: it induces their sequestration as insoluble protein aggregates and subsequently facilitates their degradation by autophagy. Aggregation of target proteins requires a distinct sequence motif in M45, which we termed 'induced protein aggregation motif'. In a second step, M45 recruits the retromer component vacuolar protein sorting 26B (VPS26B) and the microtubule-associated protein light chain 3 (LC3)-interacting adaptor protein TBC1D5 to facilitate degradation of aggregates by selective autophagy. The induced protein aggregation motif is conserved in M45-homologous proteins of several human herpesviruses, including herpes simplex virus, Epstein-Barr virus and Kaposi's sarcoma-associated herpesvirus, but is only partially conserved in the human cytomegalovirus UL45 protein. We further show that the HSV-1 ICP6 protein induces RIPK1 aggregation and degradation in a similar fashion to M45. These data suggest that induced protein aggregation combined with selective autophagy of aggregates (aggrephagy) represents a conserved viral immune-evasion mechanism.
中文翻译:

疱疹病毒诱导宿主信号蛋白NEMO和RIPK1的聚集和选择性自噬作为免疫逃逸机制。
病毒通常通过泛素-蛋白酶体途径,通过诱导关键信号转导子的降解来操纵细胞信号转导。在这里,我们显示鼠巨细胞病毒(Murid疱疹病毒1)M45蛋白诱导了两种细胞信号蛋白的降解,即活化B细胞的核因子κ-轻链增强子(NF-κB)必需调节剂(NEMO)和受体相互作用蛋白激酶1(RIPK1),通过不同的机制:以不溶性蛋白聚集体的形式诱导其螯合,并随后通过自噬促进其降解。靶蛋白的聚集需要在M45中具有独特的序列基序,我们将其称为“诱导的蛋白聚集基序”。第二步 M45募集逆转录成分的液泡蛋白分选26B(VPS26B)和与微管相关的蛋白轻链3(LC3)相互作用的衔接蛋白TBC1D5,以促进通过选择性自噬降解聚集体。诱导的蛋白质聚集基序在几种人类疱疹病毒的M45同源蛋白中保守,包括单纯疱疹病毒,爱泼斯坦-巴尔病毒和卡波西氏肉瘤相关疱疹病毒,但在人类巨细胞病毒UL45蛋白中仅部分保守。我们进一步表明,HSV-1 ICP6蛋白以与M45类似的方式诱导RIPK1聚集和降解。这些数据表明诱导的蛋白质聚集与聚集体的选择性自噬结合(蛋白吞噬)代表了保守的病毒免疫逃逸机制。
更新日期:2019-12-17
中文翻译:

疱疹病毒诱导宿主信号蛋白NEMO和RIPK1的聚集和选择性自噬作为免疫逃逸机制。
病毒通常通过泛素-蛋白酶体途径,通过诱导关键信号转导子的降解来操纵细胞信号转导。在这里,我们显示鼠巨细胞病毒(Murid疱疹病毒1)M45蛋白诱导了两种细胞信号蛋白的降解,即活化B细胞的核因子κ-轻链增强子(NF-κB)必需调节剂(NEMO)和受体相互作用蛋白激酶1(RIPK1),通过不同的机制:以不溶性蛋白聚集体的形式诱导其螯合,并随后通过自噬促进其降解。靶蛋白的聚集需要在M45中具有独特的序列基序,我们将其称为“诱导的蛋白聚集基序”。第二步 M45募集逆转录成分的液泡蛋白分选26B(VPS26B)和与微管相关的蛋白轻链3(LC3)相互作用的衔接蛋白TBC1D5,以促进通过选择性自噬降解聚集体。诱导的蛋白质聚集基序在几种人类疱疹病毒的M45同源蛋白中保守,包括单纯疱疹病毒,爱泼斯坦-巴尔病毒和卡波西氏肉瘤相关疱疹病毒,但在人类巨细胞病毒UL45蛋白中仅部分保守。我们进一步表明,HSV-1 ICP6蛋白以与M45类似的方式诱导RIPK1聚集和降解。这些数据表明诱导的蛋白质聚集与聚集体的选择性自噬结合(蛋白吞噬)代表了保守的病毒免疫逃逸机制。


























 京公网安备 11010802027423号
京公网安备 11010802027423号