当前位置:
X-MOL 学术
›
Nat. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The structure of the periplasmic FlaG-FlaF complex and its essential role for archaellar swimming motility.
Nature Microbiology ( IF 28.3 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41564-019-0622-3 Chi-Lin Tsai 1 , Patrick Tripp 2, 3 , Shamphavi Sivabalasarma 2 , Changyi Zhang 4, 5 , Marta Rodriguez-Franco 6 , Rebecca L Wipfler 4 , Paushali Chaudhury 2 , Ankan Banerjee 7 , Morgan Beeby 8 , Rachel J Whitaker 4, 5 , John A Tainer 1, 9 , Sonja-Verena Albers 2, 3
Nature Microbiology ( IF 28.3 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41564-019-0622-3 Chi-Lin Tsai 1 , Patrick Tripp 2, 3 , Shamphavi Sivabalasarma 2 , Changyi Zhang 4, 5 , Marta Rodriguez-Franco 6 , Rebecca L Wipfler 4 , Paushali Chaudhury 2 , Ankan Banerjee 7 , Morgan Beeby 8 , Rachel J Whitaker 4, 5 , John A Tainer 1, 9 , Sonja-Verena Albers 2, 3
Affiliation
|
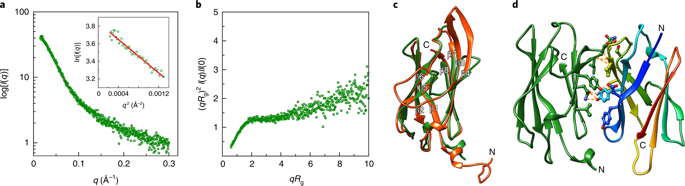
Motility structures are vital in all three domains of life. In Archaea, motility is mediated by the archaellum, a rotating type IV pilus-like structure that is a unique nanomachine for swimming motility in nature. Whereas periplasmic FlaF binds the surface layer (S-layer), the structure, assembly and roles of other periplasmic components remain enigmatic, limiting our knowledge of the archaellum's functional interactions. Here, we find that the periplasmic protein FlaG and the association with its paralogue FlaF are essential for archaellation and motility. Therefore, we determine the crystal structure of Sulfolobus acidocaldarius soluble FlaG (sFlaG), which reveals a β-sandwich fold resembling the S-layer-interacting FlaF soluble domain (sFlaF). Furthermore, we solve the sFlaG2-sFlaF2 co-crystal structure, define its heterotetrameric complex in solution by small-angle X-ray scattering and find that mutations that disrupt the complex abolish motility. Interestingly, the sFlaF and sFlaG of Pyrococcus furiosus form a globular complex, whereas sFlaG alone forms a filament, indicating that FlaF can regulate FlaG filament assembly. Strikingly, Sulfolobus cells that lack the S-layer component bound by FlaF assemble archaella but cannot swim. These collective results support a model where a FlaG filament capped by a FlaG-FlaF complex anchors the archaellum to the S-layer to allow motility.
中文翻译:

周质FlaG-FlaF复合物的结构及其对古细菌游泳运动的重要作用。
运动结构在生命的所有三个领域都至关重要。在古细菌中,运动是由古细菌介导的,古细菌是一种旋转的 IV 型菌毛状结构,是自然界中用于游泳运动的独特纳米机器。虽然周质 FlaF 结合表面层(S 层),但其他周质成分的结构、组装和作用仍然是神秘的,限制了我们对古菌功能相互作用的了解。在这里,我们发现周质蛋白 FlaG 及其与其旁系同源物 FlaF 的关联对于古细菌和运动是必不可少的。因此,我们确定了 Sulfolobus acidocaldarius 可溶性 FlaG (sFlaG) 的晶体结构,它揭示了一个类似于 S 层相互作用的 FlaF 可溶性结构域 (sFlaF) 的 β-三明治折叠。此外,我们解决了 sFlaG2-sFlaF2 共晶结构,通过小角度 X 射线散射在溶液中定义其异四聚体复合物,并发现破坏复合物的突变消除了运动性。有趣的是,Pyrococcus furiosus 的 sFlaF 和 sFlaG 形成球状复合体,而 sFlaG 单独形成细丝,表明 FlaF 可以调节 FlaG 细丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。表明FlaF可以调节FlaG灯丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。表明FlaF可以调节FlaG灯丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。
更新日期:2019-12-17
中文翻译:

周质FlaG-FlaF复合物的结构及其对古细菌游泳运动的重要作用。
运动结构在生命的所有三个领域都至关重要。在古细菌中,运动是由古细菌介导的,古细菌是一种旋转的 IV 型菌毛状结构,是自然界中用于游泳运动的独特纳米机器。虽然周质 FlaF 结合表面层(S 层),但其他周质成分的结构、组装和作用仍然是神秘的,限制了我们对古菌功能相互作用的了解。在这里,我们发现周质蛋白 FlaG 及其与其旁系同源物 FlaF 的关联对于古细菌和运动是必不可少的。因此,我们确定了 Sulfolobus acidocaldarius 可溶性 FlaG (sFlaG) 的晶体结构,它揭示了一个类似于 S 层相互作用的 FlaF 可溶性结构域 (sFlaF) 的 β-三明治折叠。此外,我们解决了 sFlaG2-sFlaF2 共晶结构,通过小角度 X 射线散射在溶液中定义其异四聚体复合物,并发现破坏复合物的突变消除了运动性。有趣的是,Pyrococcus furiosus 的 sFlaF 和 sFlaG 形成球状复合体,而 sFlaG 单独形成细丝,表明 FlaF 可以调节 FlaG 细丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。表明FlaF可以调节FlaG灯丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。表明FlaF可以调节FlaG灯丝的组装。引人注目的是,缺乏由 FlaF 结合的 S 层成分的硫化叶细胞会组装古细菌,但不能游泳。这些集体结果支持一个模型,其中由 FlaG-FlaF 复合物覆盖的 FlaG 细丝将古菌锚定到 S 层以允许运动。


























 京公网安备 11010802027423号
京公网安备 11010802027423号