Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Management of diabetic macular oedema: new insights and global implications of DRCR protocol V
Eye ( IF 3.9 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41433-019-0738-y Ning Cheung 1, 2 , Chiu Ming Gemmy Cheung 1, 2 , Steven James Talks 3 , Tien Yin Wong 1, 2
Eye ( IF 3.9 ) Pub Date : 2019-12-16 , DOI: 10.1038/s41433-019-0738-y Ning Cheung 1, 2 , Chiu Ming Gemmy Cheung 1, 2 , Steven James Talks 3 , Tien Yin Wong 1, 2
Affiliation
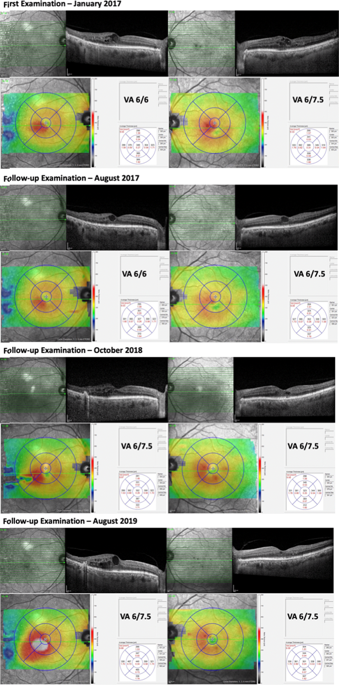
|
Over 400 million people have diabetes worldwide, with the number projected to increase close to 650 million in the next two decades [1]. In the United Kingdom alone, there are over 4 million people with diabetes [2]. As the most common microvascular complication of diabetes, diabetic retinopathy (DR) is therefore expected to remain as a global threat to vision and economy [3, 4]. Diabetic macular oedema (DMO) is now the most prevalent vision-threatening form of DR, particularly among adults with type 2 diabetes [4]. The global prevalence of DMO has been estimated to be 6.8%. Thus, about 27 million adults are affected by DMO worldwide. Fortunately, since 2010, the advent of ocular anti-vascular-endothelial-growth-factor (VEGF) therapy has greatly improved visual prognosis for patients with vision loss due to centre-involved DMO [5]. It has become the standard-of-care treatment for these patients in many developed countries [4, 6–9]. Besides DMO, ocular anti-VEGF therapy has similarly shown clear benefits for other retinal angiogenic diseases, such as proliferative DR, neovascular age-related macular degeneration, and macular oedema secondary to retinal vein occlusion [10, 11]. Its widespread use over the last two decades has undoubtedly saved sight for millions of patients worldwide. However, increasing evidence for the expanding indications of anti-VEGF therapy have also created tremendous stress to the healthcare system. Many countries are struggling to cope with the continually rising number of patients requiring this, often regular and long-term, treatment. Furthermore, this treatment burden could be compounded by clinicians who might be tempted to use the current body of evidence to assume its efficacy beyond proven clinical indications [12]. One such questionable indication was centre-involved DMO with good vision. This is an increasing issue, especially in areas with good DR screening, access to anti-VEGF therapy and increased use of optical coherence tomography in referral pathways [13]. Recently published data from the DRCR network aimed to address this important issue [14]. In the DRCR Protocol V study, three different management strategies for centre-involved DMO with good vision (visual acuity 20/25 or better) were compared in a large, adequately powered randomised clinical trial [14]. After 2 years of follow-up, there was no observable difference in visual outcome among the 702 patients who were initially managed with either aflibercept, laser or observation, with a ≥5-letter decrease in 16%, 17% and 19% respectively. Aflibercept was used when vision worsened in the laser (25%) and observation (34%) cohorts. Compared to eyes in the observation group, eyes in the laser group had a smaller chance (10% less absolute likelihood or 34% less relative likelihood) of requiring aflibercept injections. The mean visual acuity at 2 years was 20/20 in all three cohorts, and the proportions of eyes with this level of vision was 77% in the aflibercept group, 71% in the laser group, and 66% in the observation group. Collectively, these data suggest that it is generally safe to initially observe centre-involved DMO with good vision, and consider treatment only when visual impairment occurs. Although the results of the Protocol V may be unsurprising, the study does illustrate the importance of identifying whom not to treat in the era of increasing use of anti-VEGF therapy for proven and unproven indications. These data allow clinicians to feel safe to adopt a more conservative approach in the management of patients with centre-involved DMO whose vision is not yet affected (Fig. 1). As the Early Treatment Diabetic Retinopathy Study showed almost 3 decades ago, about 40% of eyes with * Tien Yin Wong wong.tien.yin@singhealth.com.sg
中文翻译:

糖尿病性黄斑水肿的管理:DRCR 方案 V 的新见解和全球影响
全世界有超过 4 亿人患有糖尿病,预计未来 20 年这一数字将增加近 6.5 亿 [1]。仅在英国,就有超过 400 万人患有糖尿病 [2]。作为糖尿病最常见的微血管并发症,因此预计糖尿病视网膜病变 (DR) 仍将是全球视力和经济的威胁 [3, 4]。糖尿病黄斑水肿 (DMO) 现在是最常见的威胁视力的 DR 形式,尤其是在 2 型糖尿病成人中 [4]。据估计,DMO 的全球流行率为 6.8%。因此,全世界约有 2700 万成年人受到 DMO 的影响。幸运的是,自 2010 年以来,眼部抗血管内皮生长因子 (VEGF) 疗法的出现大大改善了因受中心影响的 DMO 导致视力丧失的患者的视力预后 [5]。在许多发达国家,它已成为这些患者的标准治疗方法 [4, 6–9]。除了 DMO,眼部抗 VEGF 治疗也同样显示出对其他视网膜血管生成疾病的明显益处,例如增殖性 DR、新生血管性年龄相关性黄斑变性和继发于视网膜静脉阻塞的黄斑水肿 [10, 11]。它在过去二十年中的广泛使用无疑为全世界数百万患者挽救了视力。然而,越来越多的证据表明抗 VEGF 治疗的适应症不断扩大,也给医疗保健系统带来了巨大压力。许多国家都在努力应对不断增加的需要这种(通常是定期和长期)治疗的患者。此外,这种治疗负担可能会因临床医生而加剧,他们可能会倾向于使用当前的证据来假设其疗效超出已证实的临床适应症 [12]。一个这样的可疑迹象是具有良好视力的中心参与的 DMO。这是一个日益严重的问题,特别是在具有良好 DR 筛查、抗 VEGF 治疗和更多使用光学相干断层扫描的地区 [13]。最近发布的 DRCR 网络数据旨在解决这个重要问题 [14]。在 DRCR 方案 V 研究中,一项大型、充分把握的随机临床试验比较了具有良好视力(视力 20/25 或更高)的中心参与 DMO 的三种不同管理策略 [14]。经过2年的随访,在最初接受阿柏西普、激光或观察治疗的 702 名患者中,视力结果没有可观察到的差异,≥5 个字母的下降分别为 16%、17% 和 19%。当激光组 (25%) 和观察组 (34%) 视力恶化时使用阿柏西普。与观察组的眼睛相比,激光组的眼睛需要阿柏西普注射的机会较小(绝对可能性降低 10% 或相对可能性降低 34%)。所有三个队列的 2 年平均视力为 20/20,具有该视力水平的眼睛比例在阿柏西普组中为 77%,在激光组中为 71%,在观察组中为 66%。总的来说,这些数据表明,最初以良好的视力观察中心涉及的 DMO 通常是安全的,并仅在出现视力障碍时才考虑治疗。尽管方案 V 的结果可能并不令人意外,但该研究确实说明了在越来越多地使用抗 VEGF 疗法治疗已证实和未证实的适应症的时代,确定不治疗哪些人的重要性。这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg 这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg 这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg
更新日期:2019-12-16
中文翻译:

糖尿病性黄斑水肿的管理:DRCR 方案 V 的新见解和全球影响
全世界有超过 4 亿人患有糖尿病,预计未来 20 年这一数字将增加近 6.5 亿 [1]。仅在英国,就有超过 400 万人患有糖尿病 [2]。作为糖尿病最常见的微血管并发症,因此预计糖尿病视网膜病变 (DR) 仍将是全球视力和经济的威胁 [3, 4]。糖尿病黄斑水肿 (DMO) 现在是最常见的威胁视力的 DR 形式,尤其是在 2 型糖尿病成人中 [4]。据估计,DMO 的全球流行率为 6.8%。因此,全世界约有 2700 万成年人受到 DMO 的影响。幸运的是,自 2010 年以来,眼部抗血管内皮生长因子 (VEGF) 疗法的出现大大改善了因受中心影响的 DMO 导致视力丧失的患者的视力预后 [5]。在许多发达国家,它已成为这些患者的标准治疗方法 [4, 6–9]。除了 DMO,眼部抗 VEGF 治疗也同样显示出对其他视网膜血管生成疾病的明显益处,例如增殖性 DR、新生血管性年龄相关性黄斑变性和继发于视网膜静脉阻塞的黄斑水肿 [10, 11]。它在过去二十年中的广泛使用无疑为全世界数百万患者挽救了视力。然而,越来越多的证据表明抗 VEGF 治疗的适应症不断扩大,也给医疗保健系统带来了巨大压力。许多国家都在努力应对不断增加的需要这种(通常是定期和长期)治疗的患者。此外,这种治疗负担可能会因临床医生而加剧,他们可能会倾向于使用当前的证据来假设其疗效超出已证实的临床适应症 [12]。一个这样的可疑迹象是具有良好视力的中心参与的 DMO。这是一个日益严重的问题,特别是在具有良好 DR 筛查、抗 VEGF 治疗和更多使用光学相干断层扫描的地区 [13]。最近发布的 DRCR 网络数据旨在解决这个重要问题 [14]。在 DRCR 方案 V 研究中,一项大型、充分把握的随机临床试验比较了具有良好视力(视力 20/25 或更高)的中心参与 DMO 的三种不同管理策略 [14]。经过2年的随访,在最初接受阿柏西普、激光或观察治疗的 702 名患者中,视力结果没有可观察到的差异,≥5 个字母的下降分别为 16%、17% 和 19%。当激光组 (25%) 和观察组 (34%) 视力恶化时使用阿柏西普。与观察组的眼睛相比,激光组的眼睛需要阿柏西普注射的机会较小(绝对可能性降低 10% 或相对可能性降低 34%)。所有三个队列的 2 年平均视力为 20/20,具有该视力水平的眼睛比例在阿柏西普组中为 77%,在激光组中为 71%,在观察组中为 66%。总的来说,这些数据表明,最初以良好的视力观察中心涉及的 DMO 通常是安全的,并仅在出现视力障碍时才考虑治疗。尽管方案 V 的结果可能并不令人意外,但该研究确实说明了在越来越多地使用抗 VEGF 疗法治疗已证实和未证实的适应症的时代,确定不治疗哪些人的重要性。这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg 这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg 这些数据使临床医生可以安全地采用更保守的方法来管理视力尚未受到影响的中心参与的 DMO 患者(图 1)。正如近 3 年前的糖尿病视网膜病变早期治疗研究显示,大约 40% 的眼睛患有 * Tien Yin Wong wong.tien.yin@singhealth.com.sg



























 京公网安备 11010802027423号
京公网安备 11010802027423号