当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Repurposing screen identifies mebendazole as a clinical candidate to synergise with docetaxel for prostate cancer treatment.
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-17 , DOI: 10.1038/s41416-019-0681-5 Linda K Rushworth 1, 2 , Kay Hewit 2 , Sophie Munnings-Tomes 3 , Sukrut Somani 4 , Daniel James 2 , Emma Shanks 2 , Christine Dufès 4 , Anne Straube 3 , Rachana Patel 2 , Hing Y Leung 1, 2
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-17 , DOI: 10.1038/s41416-019-0681-5 Linda K Rushworth 1, 2 , Kay Hewit 2 , Sophie Munnings-Tomes 3 , Sukrut Somani 4 , Daniel James 2 , Emma Shanks 2 , Christine Dufès 4 , Anne Straube 3 , Rachana Patel 2 , Hing Y Leung 1, 2
Affiliation
|
BACKGROUND
Docetaxel chemotherapy in prostate cancer has a modest impact on survival. To date, efforts to develop combination therapies have not translated into new treatments. We sought to develop a novel therapeutic strategy to tackle chemoresistant prostate cancer by enhancing the efficacy of docetaxel.
METHODS
We performed a drug-repurposing screen by using murine-derived prostate cancer cell lines driven by clinically relevant genotypes. Cells were treated with docetaxel alone, or in combination with drugs (n = 857) from repurposing libraries, with cytotoxicity quantified using High Content Imaging Analysis.
RESULTS
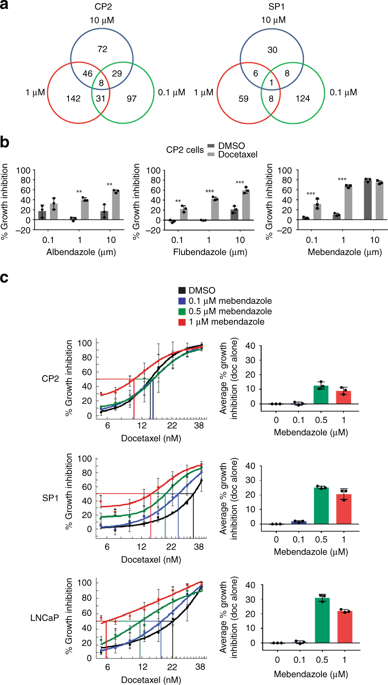
Mebendazole (an anthelmintic drug that inhibits microtubule assembly) was selected as the lead drug and shown to potently synergise docetaxel-mediated cell killing in vitro and in vivo. Dual targeting of the microtubule structure was associated with increased G2/M mitotic block and enhanced cell death. Strikingly, following combined docetaxel and mebendazole treatment, no cells divided correctly, forming multipolar spindles that resulted in aneuploid daughter cells. Liposomes entrapping docetaxel and mebendazole suppressed in vivo prostate tumour growth and extended progression-free survival.
CONCLUSIONS
Docetaxel and mebendazole target distinct aspects of the microtubule dynamics, leading to increased apoptosis and reduced tumour growth. Our data support a new concept of combined mebendazole/docetaxel treatment that warrants further clinical evaluation.
中文翻译:

重新利用筛选将甲苯咪唑确定为与多西紫杉醇协同治疗前列腺癌的临床候选药物。
背景 前列腺癌中的多西紫杉醇化学疗法对生存具有适度影响。迄今为止,开发联合疗法的努力尚未转化为新疗法。我们试图通过增强多西紫杉醇的疗效来开发一种新的治疗策略来应对化疗耐药的前列腺癌。方法 我们使用由临床相关基因型驱动的鼠源性前列腺癌细胞系进行药物再利用筛选。细胞单独使用多西紫杉醇,或与来自再利用库的药物 (n = 857) 联合使用,并使用高内涵成像分析对细胞毒性进行量化。结果 甲苯咪唑(一种抑制微管组装的驱虫药)被选为主要药物,并显示在体外和体内有效地协同多西紫杉醇介导的细胞杀伤。微管结构的双重靶向与 G2/M 有丝分裂阻滞增加和细胞死亡增加有关。引人注目的是,在多西紫杉醇和甲苯咪唑联合治疗后,没有细胞正确分裂,形成多极纺锤体,导致非整倍体子细胞。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。形成导致非整倍体子细胞的多极纺锤体。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。形成导致非整倍体子细胞的多极纺锤体。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。
更新日期:2019-12-17
中文翻译:

重新利用筛选将甲苯咪唑确定为与多西紫杉醇协同治疗前列腺癌的临床候选药物。
背景 前列腺癌中的多西紫杉醇化学疗法对生存具有适度影响。迄今为止,开发联合疗法的努力尚未转化为新疗法。我们试图通过增强多西紫杉醇的疗效来开发一种新的治疗策略来应对化疗耐药的前列腺癌。方法 我们使用由临床相关基因型驱动的鼠源性前列腺癌细胞系进行药物再利用筛选。细胞单独使用多西紫杉醇,或与来自再利用库的药物 (n = 857) 联合使用,并使用高内涵成像分析对细胞毒性进行量化。结果 甲苯咪唑(一种抑制微管组装的驱虫药)被选为主要药物,并显示在体外和体内有效地协同多西紫杉醇介导的细胞杀伤。微管结构的双重靶向与 G2/M 有丝分裂阻滞增加和细胞死亡增加有关。引人注目的是,在多西紫杉醇和甲苯咪唑联合治疗后,没有细胞正确分裂,形成多极纺锤体,导致非整倍体子细胞。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。形成导致非整倍体子细胞的多极纺锤体。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。形成导致非整倍体子细胞的多极纺锤体。包裹多西紫杉醇和甲苯咪唑的脂质体抑制了体内前列腺肿瘤的生长并延长了无进展生存期。结论 多西紫杉醇和甲苯咪唑靶向微管动力学的不同方面,导致细胞凋亡增加和肿瘤生长减少。我们的数据支持甲苯咪唑/多西紫杉醇联合治疗的新概念,需要进一步的临床评估。



























 京公网安备 11010802027423号
京公网安备 11010802027423号