当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Stable Phase Diagram of the Quaternary Water–Salt System Li+, Na+, Mg2+//SO42––H2O at T = 323 K
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.jced.9b00813 Wenzhang Zuo 1 , Ying Zeng 1, 2 , Xudong Yu 1, 2 , Wannian Ying 1 , Hanlin Tong 1 , Jingfeng Liu 1 , Pan Xu 1
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2019-12-16 , DOI: 10.1021/acs.jced.9b00813 Wenzhang Zuo 1 , Ying Zeng 1, 2 , Xudong Yu 1, 2 , Wannian Ying 1 , Hanlin Tong 1 , Jingfeng Liu 1 , Pan Xu 1
Affiliation
|
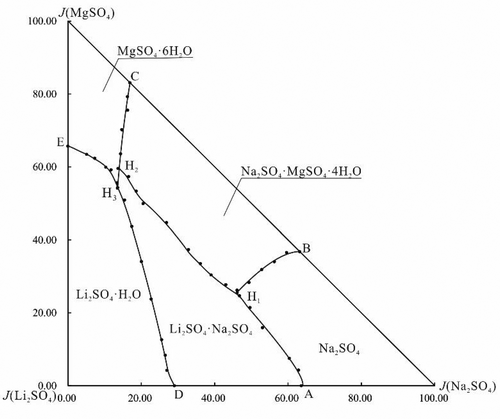
The stable equilibrium solubility, density, and refractive index of the quaternary system Li+, Na+, Mg2+//SO42––H2O were studied at T = 323 K by the isothermal dissolution method. The results show that at T = 323 K, a sodium–magnesium sulfate mineral astrakhanite (Na2SO4·MgSO4·4H2O) and a lithium–sodium sulfate double salt (Li2SO4·Na2SO4) are formed in this quaternary system 323 K, but no double salt or solid solution formed between lithium sulfate and magnesium sulfate. The phase diagram consists of seven isothermal dissolution curves, five regions of crystallization, and three invariant points, among which one belongs to an commensurate point (H3) and two belong to incommensurate points (H1 and H2). Its five crystallization fields correspond to single salts Li2SO4·H2O, Na2SO4, hexahydrite (MgSO4·6H2O), and two double salts astrakhanite (Na2SO4·MgSO4·4H2O) and Li2SO4·Na2SO4. Hexahydrite (MgSO4·6H2O) has the smallest crystal phase region, while lithium sulfate monohydrate (Li2SO4·H2O) has the largest crystal region. At equilibrium, the refractive index and density in the solution change regularly with the change in concentration of sodium sulfate. Comparisons of the phase diagrams of Li+, Na+, Mg2+//SO42––H2O at different temperatures show that the temperature is the main factor for the salt crystals, not only the crystallization form but also the crystal water content.
中文翻译:

在T = 323 K时,第四级水盐系统的稳定相图Li +,Na +,Mg 2+ // SO 4 2 – –H 2 O
通过等温溶解法研究了在T = 323 K时四元体系Li +,Na +,Mg 2+ // SO 4 2-– H 2 O的稳定平衡溶解度,密度和折射率。结果表明,在T = 323 K时,钠镁硫酸盐矿物无烟石(Na 2 SO 4 ·MgSO 4 ·4H 2 O)和锂钠硫酸钠复盐(Li 2 SO 4 ·Na 2 SO 4))在该四元体系323 K中形成,但是在硫酸锂和硫酸镁之间没有形成复盐或固溶体。相图由7条等温溶解曲线,5个结晶区域和3个不变点组成,其中1个属于等价点(H 3),另2个属于等价点(H 1和H 2)。它的五个结晶场分别对应于单盐Li 2 SO 4 ·H 2 O,Na 2 SO 4,六水合物(MgSO 4 ·6H 2 O)和两种复盐黄芪石(Na 2 SO 4 ·MgSO4 ·4H 2 O)和Li 2 SO 4 ·Na 2 SO 4。六水合物(MgSO 4 ·6H 2 O)具有最小的晶相区域,而一水合硫酸锂(Li 2 SO 4 ·H 2 O)具有最大的晶体相区域。在平衡状态下,溶液的折射率和密度随硫酸钠浓度的变化而规律地变化。Li +,Na +,Mg 2+ // SO 4 2 – –H 2相图的比较在不同温度下的O表明,温度是盐晶体的主要因素,不仅是结晶形式,而且是晶体含水量。
更新日期:2019-12-17
中文翻译:

在T = 323 K时,第四级水盐系统的稳定相图Li +,Na +,Mg 2+ // SO 4 2 – –H 2 O
通过等温溶解法研究了在T = 323 K时四元体系Li +,Na +,Mg 2+ // SO 4 2-– H 2 O的稳定平衡溶解度,密度和折射率。结果表明,在T = 323 K时,钠镁硫酸盐矿物无烟石(Na 2 SO 4 ·MgSO 4 ·4H 2 O)和锂钠硫酸钠复盐(Li 2 SO 4 ·Na 2 SO 4))在该四元体系323 K中形成,但是在硫酸锂和硫酸镁之间没有形成复盐或固溶体。相图由7条等温溶解曲线,5个结晶区域和3个不变点组成,其中1个属于等价点(H 3),另2个属于等价点(H 1和H 2)。它的五个结晶场分别对应于单盐Li 2 SO 4 ·H 2 O,Na 2 SO 4,六水合物(MgSO 4 ·6H 2 O)和两种复盐黄芪石(Na 2 SO 4 ·MgSO4 ·4H 2 O)和Li 2 SO 4 ·Na 2 SO 4。六水合物(MgSO 4 ·6H 2 O)具有最小的晶相区域,而一水合硫酸锂(Li 2 SO 4 ·H 2 O)具有最大的晶体相区域。在平衡状态下,溶液的折射率和密度随硫酸钠浓度的变化而规律地变化。Li +,Na +,Mg 2+ // SO 4 2 – –H 2相图的比较在不同温度下的O表明,温度是盐晶体的主要因素,不仅是结晶形式,而且是晶体含水量。



























 京公网安备 11010802027423号
京公网安备 11010802027423号