当前位置:
X-MOL 学术
›
Toxicol. Appl. Pharmacol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Autophagic HuR mRNA degradation induces survivin and MCL1 downregulation in YM155-treated human leukemia cells.
Toxicology and Applied Pharmacology ( IF 3.8 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.taap.2019.114857 Jing-Ting Chiou , Yuan-Chin Lee , Chia-Hui Huang , Yi-Jun Shi , Liang-Jun Wang , Long-Sen Chang
Toxicology and Applied Pharmacology ( IF 3.8 ) Pub Date : 2019-12-16 , DOI: 10.1016/j.taap.2019.114857 Jing-Ting Chiou , Yuan-Chin Lee , Chia-Hui Huang , Yi-Jun Shi , Liang-Jun Wang , Long-Sen Chang
|
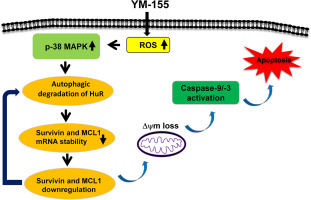
The aim of this study was to investigate the mechanism of YM155 cytotoxicity in human chronic myeloid leukemia (CML) cells. YM155-induced apoptosis of human CML K562 cells was characterized by ROS-mediated p38 MAPK activation, mitochondrial depolarization, and survivin and MCL1 downregulation. Moreover, YM155-induced autophagy caused degradation of HuR mRNA and downregulation of HuR protein expression, which resulted in destabilized survivin and MCL1 mRNA. Interestingly, survivin and MCL1 suppression contributed to autophagy-mediated HuR mRNA destabilization in YM155-treated cells. Pretreatment with inhibitors of p38 MAPK or autophagy alleviated YM155-induced autophagy and apoptosis in K562 cells, as well as YM155-induced downregulation of HuR, survivin, and MCL1. Ectopic overexpression of HuR, survivin, or MCL1 attenuated the cytotoxic effect of YM155 on K562 cells. Conversely, YM155 sensitized K562 cells to ABT-199 (a BCL2 inhibitor), and circumvented K562 cell resistance to ABT-199 because of its inhibitory effect on survivin and MCL1 expression. Overall, our data indicate that YM155-induced apoptosis is mediated by inducing autophagic HuR mRNA degradation, and reveal the pathway responsible for YM155-induced downregulation of survivin and MCL1 in K562 cells. Our findings also indicate a similar pathway underlying YM155-induced death in human CML MEG-01 cells.
中文翻译:

自噬 HuR mRNA 降解诱导 YM155 处理的人类白血病细胞中的生存素和 MCL1 下调。
本研究的目的是研究 YM155 对人慢性粒细胞白血病 (CML) 细胞的细胞毒性机制。YM155 诱导的人 CML K562 细胞凋亡的特征在于 ROS 介导的 p38 MAPK 激活、线粒体去极化以及存活素和 MCL1 下调。此外,YM155 诱导的自噬导致 HuR mRNA 降解和 HuR 蛋白表达下调,导致 survivin 和 MCL1 mRNA 不稳定。有趣的是,survivin 和 MCL1 抑制导致 YM155 处理的细胞中自噬介导的 HuR mRNA 失稳。用 p38 MAPK 或自噬抑制剂预处理可减轻 YM155 诱导的 K562 细胞自噬和凋亡,以及 YM155 诱导的 HuR、survivin 和 MCL1 下调。HuR、survivin 的异位过表达,或 MCL1 减弱 YM155 对 K562 细胞的细胞毒作用。相反,YM155 使 K562 细胞对 ABT-199(一种 BCL2 抑制剂)敏感,并由于其对生存素和 MCL1 表达的抑制作用而规避了 K562 细胞对 ABT-199 的抗性。总体而言,我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导的 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。
更新日期:2019-12-17
中文翻译:

自噬 HuR mRNA 降解诱导 YM155 处理的人类白血病细胞中的生存素和 MCL1 下调。
本研究的目的是研究 YM155 对人慢性粒细胞白血病 (CML) 细胞的细胞毒性机制。YM155 诱导的人 CML K562 细胞凋亡的特征在于 ROS 介导的 p38 MAPK 激活、线粒体去极化以及存活素和 MCL1 下调。此外,YM155 诱导的自噬导致 HuR mRNA 降解和 HuR 蛋白表达下调,导致 survivin 和 MCL1 mRNA 不稳定。有趣的是,survivin 和 MCL1 抑制导致 YM155 处理的细胞中自噬介导的 HuR mRNA 失稳。用 p38 MAPK 或自噬抑制剂预处理可减轻 YM155 诱导的 K562 细胞自噬和凋亡,以及 YM155 诱导的 HuR、survivin 和 MCL1 下调。HuR、survivin 的异位过表达,或 MCL1 减弱 YM155 对 K562 细胞的细胞毒作用。相反,YM155 使 K562 细胞对 ABT-199(一种 BCL2 抑制剂)敏感,并由于其对生存素和 MCL1 表达的抑制作用而规避了 K562 细胞对 ABT-199 的抗性。总体而言,我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导的 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。我们的数据表明 YM155 诱导的细胞凋亡是通过诱导自噬 HuR mRNA 降解介导的,并揭示了 YM155 诱导 K562 细胞中 survivin 和 MCL1 下调的途径。我们的研究结果还表明 YM155 诱导的人类 CML MEG-01 细胞死亡的类似途径。



























 京公网安备 11010802027423号
京公网安备 11010802027423号