当前位置:
X-MOL 学术
›
Chemistryopen
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Electrochemical Oxidation of Sulfonamides with Boron-Doped Diamond and Pt Anodes.
ChemistryOpen ( IF 2.3 ) Pub Date : 2019-12-13 , DOI: 10.1002/open.201900250 Hongna Li 1 , Huan Jiang 2 , Chong Liu 1 , Changxiong Zhu 1 , Xiuping P Zhu 3
ChemistryOpen ( IF 2.3 ) Pub Date : 2019-12-13 , DOI: 10.1002/open.201900250 Hongna Li 1 , Huan Jiang 2 , Chong Liu 1 , Changxiong Zhu 1 , Xiuping P Zhu 3
Affiliation
|
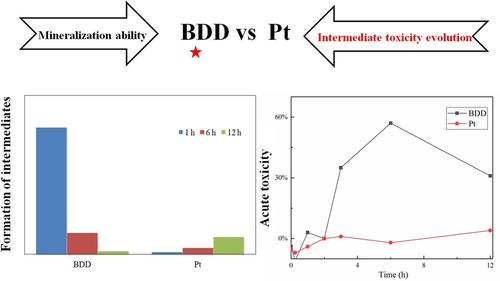
Electrochemical oxidation processes usually favored specific degradation pathways depending on anode materials. In this work, a series of sulfonamides (SNs) were degraded by electrochemical oxidation. Compared to Pt anodes (0.1567–0.1795 h−1), degradation rates of SNs were much higher at boron‐doped diamond (BDD) anodes (2.4290–13.1950 h−1). However, the same intermediates were detected in the two anode systems. Due to the strong oxidizing ability of BDD anodes, a large amount of intermediates with high toxicities were initially generated and then finally reduced in the BDD anode systems, while the amount of intermediates continuously increased in the Pt anode systems. Additionally, SNs were degraded faster in Na2SO4 than NaH2PO4 electrolytes at BDD anodes, while they were similar at Pt anodes. This study demonstrated that the degradation pathways of SNs at BDD and Pt anodes were similar, but the evolutions of intermediate amounts and toxicities were different due to their varied oxidizing abilities.
中文翻译:

掺硼金刚石和铂阳极对磺酰胺的电化学氧化。
取决于阳极材料,电化学氧化过程通常有利于特定的降解途径。在这项工作中,一系列的磺酰胺(SN)被电化学氧化降解。与Pt阳极(0.1567–0.1795 h -1)相比,SN的降解率在掺硼金刚石(BDD)阳极(2.4290–13.1950 h -1)要高得多。但是,在两个阳极系统中检测到相同的中间体。由于BDD阳极具有很强的氧化能力,因此在BDD阳极系统中首先生成了大量具有高毒性的中间体,然后最终将其还原,而在Pt阳极系统中则不断增加中间体的数量。此外,SNs在Na 2 SO 4中的降解速度要比NaH快BDD阳极处的2 PO 4电解质,而Pt阳极处的电解质相似。这项研究表明,BDD和Pt阳极上SN的降解途径是相似的,但是由于它们的不同的氧化能力,中间量和毒性的演变是不同的。
更新日期:2019-12-13
中文翻译:

掺硼金刚石和铂阳极对磺酰胺的电化学氧化。
取决于阳极材料,电化学氧化过程通常有利于特定的降解途径。在这项工作中,一系列的磺酰胺(SN)被电化学氧化降解。与Pt阳极(0.1567–0.1795 h -1)相比,SN的降解率在掺硼金刚石(BDD)阳极(2.4290–13.1950 h -1)要高得多。但是,在两个阳极系统中检测到相同的中间体。由于BDD阳极具有很强的氧化能力,因此在BDD阳极系统中首先生成了大量具有高毒性的中间体,然后最终将其还原,而在Pt阳极系统中则不断增加中间体的数量。此外,SNs在Na 2 SO 4中的降解速度要比NaH快BDD阳极处的2 PO 4电解质,而Pt阳极处的电解质相似。这项研究表明,BDD和Pt阳极上SN的降解途径是相似的,但是由于它们的不同的氧化能力,中间量和毒性的演变是不同的。


























 京公网安备 11010802027423号
京公网安备 11010802027423号