当前位置:
X-MOL 学术
›
Protein Expres. Purif.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cloning, expression and characterization of a thermo-alkali-stable xylanase from Aspergillus oryzae LC1 in Escherichia coli BL21(DE3).
Protein Expression and Purification ( IF 1.6 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.pep.2019.105551 Nisha Bhardwaj 1 , Vijay Kumar Verma 1 , Venkatesh Chaturvedi 2 , Pradeep Verma 1
Protein Expression and Purification ( IF 1.6 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.pep.2019.105551 Nisha Bhardwaj 1 , Vijay Kumar Verma 1 , Venkatesh Chaturvedi 2 , Pradeep Verma 1
Affiliation
|
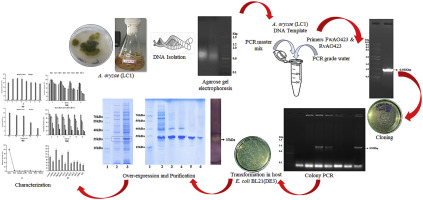
In the present investigation, cloning and overexpression of xylanase (XynF1), the main xylanase of A. oryzae LC1, was performed in prokaryotic system E. coli BL21(DE3) to produce recombinant xylanase with high titer of specific activity (1037.3 U/mg), which was 9.3-fold higher than the native strain. Further, the recombinant XynF1 of size 37 kDa was purified using Ni2+-NTA resins followed by cation exchange chromatography, which showed an 1.8-fold increase in purity with 71.4% yield. The r-XynF1 exhibited a wide range of activity at different pH (3.0-10.0) range and temperature (30-70 °C) with an optimum pH at 5.0 and temperature at 30 °C. The results from the current study, clearly demonstrate that this is an effective method to generate a recombinant enzyme with improved activity, making it useful for possible industrial applications.
中文翻译:

水稻BL21(DE3)中米曲霉LC1的热碱稳定木聚糖酶的克隆,表达和鉴定。
在本研究中,米曲霉LC1的主要木聚糖酶木聚糖酶(XynF1)的克隆和过表达在原核系统大肠杆菌BL21(DE3)中进行,以生产具有高滴度比活性(1037.3 U / mg的重组木聚糖酶)。 ),比天然菌株高9.3倍。此外,使用Ni2 + -NTA树脂和阳离子交换色谱法纯化了大小为37 kDa的重组XynF1,其纯度提高了1.8倍,产率为71.4%。r-XynF1在不同的pH(3.0-10.0)范围和温度(30-70°C)下表现出广泛的活性,在5.0和30°C的温度下具有最佳的pH值。当前研究的结果清楚地表明,这是产生具有改进活性的重组酶的有效方法,使其可用于可能的工业应用。
更新日期:2019-12-13
中文翻译:

水稻BL21(DE3)中米曲霉LC1的热碱稳定木聚糖酶的克隆,表达和鉴定。
在本研究中,米曲霉LC1的主要木聚糖酶木聚糖酶(XynF1)的克隆和过表达在原核系统大肠杆菌BL21(DE3)中进行,以生产具有高滴度比活性(1037.3 U / mg的重组木聚糖酶)。 ),比天然菌株高9.3倍。此外,使用Ni2 + -NTA树脂和阳离子交换色谱法纯化了大小为37 kDa的重组XynF1,其纯度提高了1.8倍,产率为71.4%。r-XynF1在不同的pH(3.0-10.0)范围和温度(30-70°C)下表现出广泛的活性,在5.0和30°C的温度下具有最佳的pH值。当前研究的结果清楚地表明,这是产生具有改进活性的重组酶的有效方法,使其可用于可能的工业应用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号