当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis, biological evaluation and anti-proliferative mechanism of fluorine-containing proguanil derivatives.
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.bmc.2019.115258 Di Xiao 1 , Zhicheng Lu 2 , Zhiren Wang 1 , Sichun Zhou 1 , Mengru Cao 3 , Jun Deng 1 , Xin Hu 1 , Mei Peng 1 , Caimei He 1 , Jingtao Wu 1 , Simeng Xu 1 , Huihui Zhang 1 , Cangcang Xu 1 , Wei Wang 3 , Aiying Guan 2 , Xiaoping Yang 1
Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2019-12-12 , DOI: 10.1016/j.bmc.2019.115258 Di Xiao 1 , Zhicheng Lu 2 , Zhiren Wang 1 , Sichun Zhou 1 , Mengru Cao 3 , Jun Deng 1 , Xin Hu 1 , Mei Peng 1 , Caimei He 1 , Jingtao Wu 1 , Simeng Xu 1 , Huihui Zhang 1 , Cangcang Xu 1 , Wei Wang 3 , Aiying Guan 2 , Xiaoping Yang 1
Affiliation
|
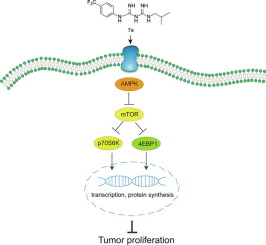
Proguanil, a member of biguanide family, has excellent anti-proliferative activities. Fluorine-containing compounds have been demonstrated to have super biological activities including enhanced binding interactions, metabolic stability, and reduced toxicity. In this study, based on the intermediate derivatization methods, we synthesized 13 new fluorine-containing proguanil derivatives, and found that 7a,7d and 8e had much lower IC50 than proguanil in 5 human cancerous cell lines. The results of clonogenic and scratch wound healing assays revealed that the inhibitory effects of derivatives 7a,7d and 8e on proliferation and migration of human cancer cell lines were much better than proguanil as well. Mechanistic study based on representative derivative 7a indicated that this compound up-regulates AMPK signal pathway and downregulates mTOR/4EBP1/p70S6K. In conclusion, these new fluorine-containing derivatives show potential for the development of cancer chemotherapeutic drugs.
中文翻译:

含氟鸟嘌呤衍生物的合成,生物学评价和抗增殖机理。
双胍家族的成员Proguanil具有出色的抗增殖活性。已证明含氟化合物具有超强的生物活性,包括增强的结合相互作用,代谢稳定性和降低的毒性。在这项研究中,基于中间衍生化方法,我们合成了13种新的含氟鸟嘌呤衍生物,发现7a,7d和8e在5种人类癌细胞系中的IC50远低于鸟嘌呤。克隆形成和划痕伤口愈合试验的结果表明,衍生物7a,7d和8e对人癌细胞系增殖和迁移的抑制作用也远优于鸟嘌呤。基于代表性衍生物7a的机理研究表明,该化合物上调AMPK信号通路,下调mTOR / 4EBP1 / p70S6K。总之,这些新的含氟衍生物显示出开发癌症化疗药物的潜力。
更新日期:2019-12-13
中文翻译:

含氟鸟嘌呤衍生物的合成,生物学评价和抗增殖机理。
双胍家族的成员Proguanil具有出色的抗增殖活性。已证明含氟化合物具有超强的生物活性,包括增强的结合相互作用,代谢稳定性和降低的毒性。在这项研究中,基于中间衍生化方法,我们合成了13种新的含氟鸟嘌呤衍生物,发现7a,7d和8e在5种人类癌细胞系中的IC50远低于鸟嘌呤。克隆形成和划痕伤口愈合试验的结果表明,衍生物7a,7d和8e对人癌细胞系增殖和迁移的抑制作用也远优于鸟嘌呤。基于代表性衍生物7a的机理研究表明,该化合物上调AMPK信号通路,下调mTOR / 4EBP1 / p70S6K。总之,这些新的含氟衍生物显示出开发癌症化疗药物的潜力。

























 京公网安备 11010802027423号
京公网安备 11010802027423号