当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural Basis for Finding OG Lesions and Avoiding Undamaged G by the DNA Glycosylase MutY.
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-12-27 , DOI: 10.1021/acschembio.9b00639 L Peyton Russelburg 1 , Valerie L O'Shea Murray 2, 3 , Merve Demir 2 , Kyle R Knutsen 1 , Sonia L Sehgal 1 , Sheng Cao 2 , Sheila S David 2 , Martin P Horvath 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2019-12-27 , DOI: 10.1021/acschembio.9b00639 L Peyton Russelburg 1 , Valerie L O'Shea Murray 2, 3 , Merve Demir 2 , Kyle R Knutsen 1 , Sonia L Sehgal 1 , Sheng Cao 2 , Sheila S David 2 , Martin P Horvath 1
Affiliation
|
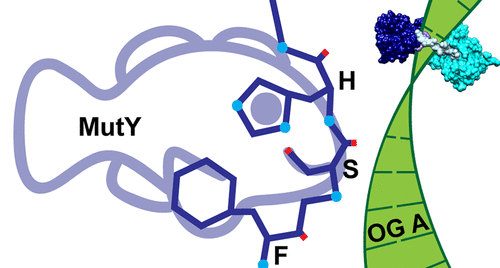
The adenine glycosylase MutY selectively initiates repair of OG:A lesions and, by comparison, avoids G:A mispairs. The ability to distinguish these closely related substrates relies on the C-terminal domain of MutY, which structurally resembles MutT. To understand the mechanism for substrate specificity, we crystallized MutY in complex with DNA containing G across from the high-affinity azaribose transition state analogue. Our structure shows that G is accommodated by the OG site and highlights the role of a serine residue in OG versus G discrimination. The functional significance of Ser308 and its neighboring residues was evaluated by mutational analysis, revealing the critical importance of a β loop in the C-terminal domain for mutation suppression in cells, and biochemical performance in vitro. This loop comprising residues Phe307, Ser308, and His309 (Geobacillus stearothermophilus sequence positions) is conserved in MutY but absent in MutT and other DNA repair enzymes and may therefore serve as a MutY-specific target exploitable by chemical biological probes.
中文翻译:

寻找OG病变并避免DNA糖基化酶MutY损坏G的结构基础。
腺嘌呤糖基化酶MutY选择性地启动OG:A病变的修复,相比之下,避免了G:A错配。区分这些密切相关的底物的能力取决于MutY的C末端结构域,该结构在结构上类似于MutT。为了了解底物特异性的机制,我们将MutY与高亲和性阿里巴糖过渡态类似物对面的含G的DNA复合形成了结晶。我们的结构表明,OG位点可容纳G,并突显了丝氨酸残基在OG与G区分中的作用。通过突变分析评估了Ser308及其邻近残基的功能意义,揭示了C末端结构域中的β环对于细胞中的突变抑制以及体外生化性能至关重要。该环包含残基Phe307,Ser308,
更新日期:2019-12-29
中文翻译:

寻找OG病变并避免DNA糖基化酶MutY损坏G的结构基础。
腺嘌呤糖基化酶MutY选择性地启动OG:A病变的修复,相比之下,避免了G:A错配。区分这些密切相关的底物的能力取决于MutY的C末端结构域,该结构在结构上类似于MutT。为了了解底物特异性的机制,我们将MutY与高亲和性阿里巴糖过渡态类似物对面的含G的DNA复合形成了结晶。我们的结构表明,OG位点可容纳G,并突显了丝氨酸残基在OG与G区分中的作用。通过突变分析评估了Ser308及其邻近残基的功能意义,揭示了C末端结构域中的β环对于细胞中的突变抑制以及体外生化性能至关重要。该环包含残基Phe307,Ser308,


























 京公网安备 11010802027423号
京公网安备 11010802027423号