当前位置:
X-MOL 学术
›
ACS Cent. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Quantitatively Unraveling the Redox Shuttle of Spontaneous Oxidation/Electroreduction of CuO x on Silver Nanowires Using in Situ X-ray Absorption Spectroscopy.
ACS Central Science ( IF 18.2 ) Pub Date : 2019-12-11 , DOI: 10.1021/acscentsci.9b01142 Chia-Jui Chang,Sung-Fu Hung,Chia-Shuo Hsu,Hsiao-Chien Chen,Sheng-Chih Lin,Yen-Fa Liao,Hao Ming Chen
ACS Central Science ( IF 18.2 ) Pub Date : 2019-12-11 , DOI: 10.1021/acscentsci.9b01142 Chia-Jui Chang,Sung-Fu Hung,Chia-Shuo Hsu,Hsiao-Chien Chen,Sheng-Chih Lin,Yen-Fa Liao,Hao Ming Chen
|
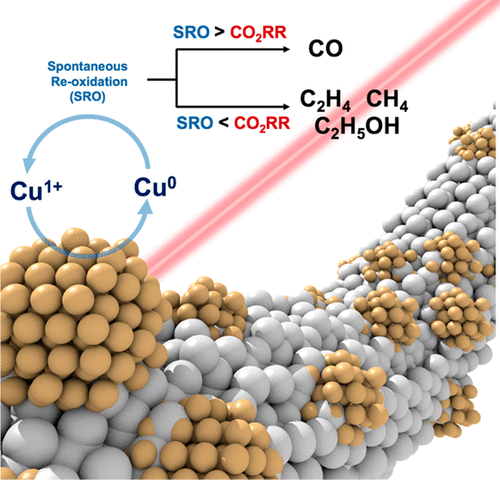
Oxide-derived copper catalysts have been shown to enhance CO2 reduction reaction (CO2RR) activity with high selectivity toward hydrocarbon products. However, the chemical state of oxide-derived copper during the CO2RR has remained elusive and is lacking in situ observations. Herein, a two-step process was developed to synthesize Ag nanowires coated with various thicknesses of a CuO x layer for the CO2RR. By employing in situ X-ray absorption spectroscopy, a strong correlation between the chemical state under reaction conditions and the CO2RR product profile can be revealed to validate another competing reaction (i.e., the spontaneous oxidation of Cu(0) in aqueous electrolyte) that significantly governs the chemical state of active centers of Cu. In situ Raman spectroscopy reveals the existence of reoxidation behavior under cathodic potential, and the quantification analysis of reoxidized behavior is revealed to indicate that the reoxidation rate is independent of surface morphology and strongly proportional to the electrochemically surface area. The steady oxidation state of Cu in an in situ condition is the paramount key and dominates the products' profile of the CO2RR rather than other factors (e.g., crystal facets, atomic arrangements, morphology, elements) that have been investigated in numerous reports.
中文翻译:

使用原位X射线吸收光谱法定量解开银纳米线上CuO x的自发氧化/电还原的氧化还原梭。
氧化物衍生的铜催化剂已显示出可提高CO2还原反应(CO2RR)活性,并且对烃类产品具有高选择性。但是,CO2RR过程中源自氧化物的铜的化学状态仍然难以捉摸,并且缺乏现场观察。本文中,开发了两步过程以合成涂覆有各种厚度的CO2RR的CuO x层的Ag纳米线。通过使用原位X射线吸收光谱,可以揭示反应条件下的化学状态与CO2RR产物分布之间的强相关性,以验证另一种竞争性反应(即,水性电解质中Cu(0)的自发氧化)控制铜的活性中心的化学状态。原位拉曼光谱揭示了在阴极电势下再氧化行为的存在,对再氧化行为的定量分析表明,再氧化速率与表面形态无关,与电化学表面积成正比。处于原位状态的Cu的稳定氧化状态是最重要的,它决定了CO2RR的产品分布,而不是其他许多报告中已经研究过的其他因素(例如,晶面,原子排列,形态,元素)。
更新日期:2019-12-27
中文翻译:

使用原位X射线吸收光谱法定量解开银纳米线上CuO x的自发氧化/电还原的氧化还原梭。
氧化物衍生的铜催化剂已显示出可提高CO2还原反应(CO2RR)活性,并且对烃类产品具有高选择性。但是,CO2RR过程中源自氧化物的铜的化学状态仍然难以捉摸,并且缺乏现场观察。本文中,开发了两步过程以合成涂覆有各种厚度的CO2RR的CuO x层的Ag纳米线。通过使用原位X射线吸收光谱,可以揭示反应条件下的化学状态与CO2RR产物分布之间的强相关性,以验证另一种竞争性反应(即,水性电解质中Cu(0)的自发氧化)控制铜的活性中心的化学状态。原位拉曼光谱揭示了在阴极电势下再氧化行为的存在,对再氧化行为的定量分析表明,再氧化速率与表面形态无关,与电化学表面积成正比。处于原位状态的Cu的稳定氧化状态是最重要的,它决定了CO2RR的产品分布,而不是其他许多报告中已经研究过的其他因素(例如,晶面,原子排列,形态,元素)。


























 京公网安备 11010802027423号
京公网安备 11010802027423号