当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Biosynthesis of the N-N-Bond-Containing Compound l-Alanosine.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201913458 Menghua Wang 1 , Haruka Niikura 1 , Hai-Yan He 1 , Phillip Daniel-Ivad 1 , Katherine S Ryan 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/anie.201913458 Menghua Wang 1 , Haruka Niikura 1 , Hai-Yan He 1 , Phillip Daniel-Ivad 1 , Katherine S Ryan 1
Affiliation
|
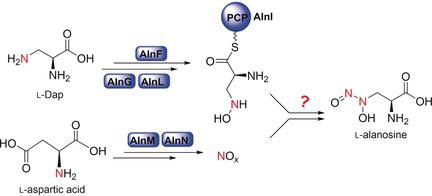
The formation of a N-N bond is a unique biochemical transformation, and nature employs diverse biosynthetic strategies to activate nitrogen for bond formation. Among molecules that contain a N-N bond, biosynthetic routes to diazeniumdiolates remain enigmatic. We here report the biosynthetic pathway for the diazeniumdiolate-containing amino acid l-alanosine. Our work reveals that the two nitrogen atoms in the diazeniumdiolate of l-alanosine arise from glutamic acid and aspartic acid, and we clarify the early steps of the biosynthetic pathway by using both in vitro and in vivo approaches. Our work demonstrates a peptidyl-carrier-protein-based mechanism for activation of the precursor l-diaminopropionate, and we also show that nitric oxide can participate in non-enzymatic diazeniumdiolate formation. Furthermore, we demonstrate that the gene alnA, which encodes a fusion protein with an N-terminal cupin domain and a C-terminal AraC-like DNA-binding domain, is required for alanosine biosynthesis.
中文翻译:

含NN键的化合物I-Alanosine的生物合成。
NN键的形成是独特的生化转变,自然界采用多种生物合成策略来活化氮以形成键。在含有NN键的分子中,二氮杂二烯二醇盐的生物合成途径仍然是个谜。我们在这里报告了含重氮二醇二乙酸的氨基酸l-丙氨酸的生物合成途径。我们的工作表明,L-丙氨酸的二氮杂二氮烯齿酸酯中的两个氮原子来自谷氨酸和天冬氨酸,并且我们通过使用体内和体外方法阐明了生物合成途径的早期步骤。我们的工作证明了基于肽基载体蛋白的激活前体1-二氨基丙酸酯的机制,并且我们还表明一氧化氮可以参与非酶促二氮烯二氮杂酸酯的形成。此外,我们证明了alnA基因,
更新日期:2020-01-23
中文翻译:

含NN键的化合物I-Alanosine的生物合成。
NN键的形成是独特的生化转变,自然界采用多种生物合成策略来活化氮以形成键。在含有NN键的分子中,二氮杂二烯二醇盐的生物合成途径仍然是个谜。我们在这里报告了含重氮二醇二乙酸的氨基酸l-丙氨酸的生物合成途径。我们的工作表明,L-丙氨酸的二氮杂二氮烯齿酸酯中的两个氮原子来自谷氨酸和天冬氨酸,并且我们通过使用体内和体外方法阐明了生物合成途径的早期步骤。我们的工作证明了基于肽基载体蛋白的激活前体1-二氨基丙酸酯的机制,并且我们还表明一氧化氮可以参与非酶促二氮烯二氮杂酸酯的形成。此外,我们证明了alnA基因,


























 京公网安备 11010802027423号
京公网安备 11010802027423号