JAMA Surgery ( IF 16.9 ) Pub Date : 2019-12-11 , DOI: 10.1001/jamasurg.2019.4913 Steven B Porter 1 , Amy E Glasgow 2 , Xiaoxi Yao 2 , Elizabeth B Habermann 2, 3
|
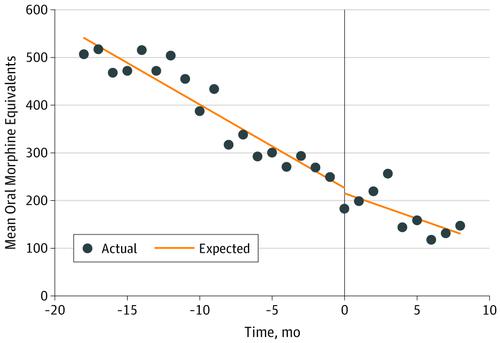
In 2017, more than 47 000 Americans died of opioid misuse and overdose.1 In response to this crisis, many states have signed into law new regulations regarding the prescribing of opioids for acute and chronic pain. Florida House Bill 21 (HB21),2 which went into effect on July 1, 2018, mandated the following changes (among others) to opioid prescribing: (1) a limit of a 3-day to 7-day supply of opioids for acute pain, (2) a prohibition of refills ordered with the initial opioid prescription for acute pain, and (3) a requirement that the prescribing physician or his or her delegate check Florida’s prescription drug–monitoring program prior to prescribing opioids. We sought to quantify the association of Florida HB21 with opioid prescribing for acute postoperative pain at our institution.
中文翻译:

佛罗里达州众议院第21号法案与单一机构接受术后阿片类药物急性疼痛处方的协会。
2017年,超过47 000名美国人死于阿片类药物滥用和药物过量。[1]为应对这一危机,许多州已将有关处方阿片类药物用于急性和慢性疼痛的新法规签署为法律。佛罗里达众议院法案21(HB21),2该药品于2018年7月1日生效,其中规定对阿片类药物处方进行以下更改(其中包括):( 1)限制3天至7天的阿片类药物急性疼痛供应;(2)禁止补充下达了用于急性疼痛的初始阿片类药物处方,以及(3)要求开处方的医生或其委托人在开具阿片类药物之前检查佛罗里达州的处方药监测计划。我们试图量化佛罗里达州HB21与阿片类药物处方在我们机构中的急性术后疼痛的关系。


























 京公网安备 11010802027423号
京公网安备 11010802027423号