当前位置:
X-MOL 学术
›
npj Parkinsons Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
PINK1 phosphorylates ubiquitin predominantly in astrocytes.
npj Parkinson's Disease ( IF 9.304 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41531-019-0101-9 Sandeep K Barodia 1 , Laura J McMeekin 2, 3 , Rose B Creed 1 , Elijah K Quinones 1 , Rita M Cowell 2, 3 , Matthew S Goldberg 1, 4
npj Parkinson's Disease ( IF 9.304 ) Pub Date : 2019-12-11 , DOI: 10.1038/s41531-019-0101-9 Sandeep K Barodia 1 , Laura J McMeekin 2, 3 , Rose B Creed 1 , Elijah K Quinones 1 , Rita M Cowell 2, 3 , Matthew S Goldberg 1, 4
Affiliation
|
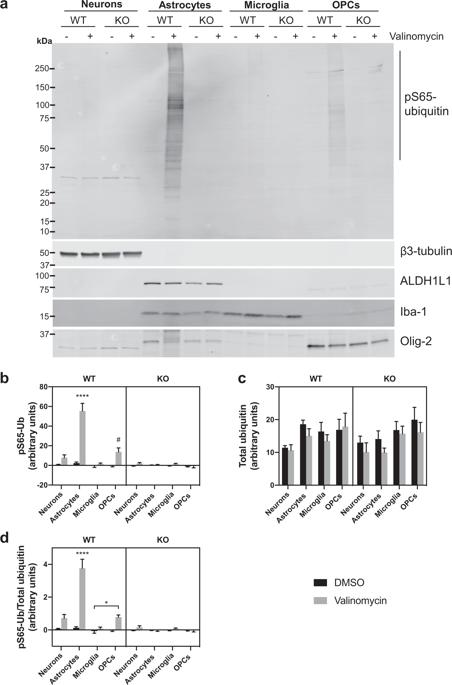
Loss-of-function mutations in PINK1 are causally linked to recessively inherited Parkinson's disease (PD), with marked loss of dopaminergic neurons in the substantia nigra that are required for normal movement. PINK1 is a nuclear-encoded mitochondrial-targeted kinase that phosphorylates a conserved serine at amino acid 65 (pS65) in ubiquitin as well as Parkin, another gene with loss-of-function mutations linked to recessive parkinsonism. The steady-state levels of PINK1 protein are very low, even in cells that express PINK1, because PINK1 is normally targeted for degradation after mitochondrial import by a process that is dependent upon mitochondrial membrane potential. Dissipation of the mitochondrial membrane potential with ionophores, such as CCCP and valinomycin, causes the accumulation of PINK1 on the outer mitochondrial membrane, a marked increase of pS65-ubiquitin and the recruitment of Parkin, which targets dysfunctional mitochondria for degradation by autophagy. While the high penetrance of PINK1 mutations establish its critical function for maintaining neurons, the activity of PINK1 in primary neurons has been difficult to detect. Mounting evidence implicates non-neuronal cells, including astrocytes and microglia, in the pathogenesis of both idiopathic and inherited PD. Herein we used both western analysis and immunofluorescence of pS65-ubiquitin to directly compare the activity of PINK1 in primary neurons, astrocytes, microglia, and oligodendrocyte progenitor cells cultured from the brains of wild-type (WT) and PINK1 knockout (KO) rat pups. Our findings that PINK1-dependent ubiquitin phosphorylation is predominantly in astrocytes supports increased priority for research on the function of PINK1 in astrocytes and the contribution of astrocyte dysfunction to PD pathogenesis.
中文翻译:

PINK1主要使星形胶质细胞中的泛素磷酸化。
PINK1的功能丧失突变与隐性遗传的帕金森氏病(PD)有因果关系,黑质中的多巴胺能神经元明显丧失,这是正常运动所必需的。PINK1是一种核编码的线粒体靶向激酶,可在泛素和帕金(一种与隐性帕金森病相关的功能丧失突变的基因)中磷酸化保守的65位氨基酸(pS65)上的保守丝氨酸。即使在表达PINK1的细胞中,PINK1蛋白的稳态水平也非常低,因为PINK1通常是通过依赖于线粒体膜电位的过程在线粒体导入后降解的靶标。离子载体(例如CCCP和缬氨霉素)耗散线粒体膜电位会导致PINK1在线粒体外膜上积聚,pS65泛素的显着增加和Parkin的募集,后者靶向功能障碍的线粒体以通过自噬降解。尽管PINK1突变的高渗透性确立了其维持神经元的关键功能,但很难检测到初级神经元中PINK1的活性。越来越多的证据表明,特发性和遗传性PD的发病机理都涉及非神经元细胞,包括星形胶质细胞和小胶质细胞。本文中,我们使用了Western分析和pS65泛素的免疫荧光技术,直接比较了从野生型(WT)和PINK1敲除(KO)大鼠幼犬的大脑培养的原代神经元,星形胶质细胞,小胶质细胞和少突胶质祖细胞中PINK1的活性。 。
更新日期:2019-12-11
中文翻译:

PINK1主要使星形胶质细胞中的泛素磷酸化。
PINK1的功能丧失突变与隐性遗传的帕金森氏病(PD)有因果关系,黑质中的多巴胺能神经元明显丧失,这是正常运动所必需的。PINK1是一种核编码的线粒体靶向激酶,可在泛素和帕金(一种与隐性帕金森病相关的功能丧失突变的基因)中磷酸化保守的65位氨基酸(pS65)上的保守丝氨酸。即使在表达PINK1的细胞中,PINK1蛋白的稳态水平也非常低,因为PINK1通常是通过依赖于线粒体膜电位的过程在线粒体导入后降解的靶标。离子载体(例如CCCP和缬氨霉素)耗散线粒体膜电位会导致PINK1在线粒体外膜上积聚,pS65泛素的显着增加和Parkin的募集,后者靶向功能障碍的线粒体以通过自噬降解。尽管PINK1突变的高渗透性确立了其维持神经元的关键功能,但很难检测到初级神经元中PINK1的活性。越来越多的证据表明,特发性和遗传性PD的发病机理都涉及非神经元细胞,包括星形胶质细胞和小胶质细胞。本文中,我们使用了Western分析和pS65泛素的免疫荧光技术,直接比较了从野生型(WT)和PINK1敲除(KO)大鼠幼犬的大脑培养的原代神经元,星形胶质细胞,小胶质细胞和少突胶质祖细胞中PINK1的活性。 。

























 京公网安备 11010802027423号
京公网安备 11010802027423号