Trends in Environmental Analytical Chemistry ( IF 11.2 ) Pub Date : 2019-07-13 , DOI: 10.1016/j.teac.2019.e00069 Elisabeth Cuervo Lumbaque , Elaine R. Lopes Tiburtius , Márcio Barreto-Rodrigues , Carla Sirtori
|
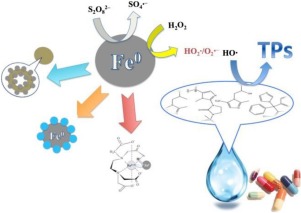
Zero-valent iron (Fe0) has recently been proposed as a potential candidate for the degradation of pharmaceuticals, because Fe0 can release dissolved iron species, activate molecular oxygen, and react with oxidant species. Additionally, due to its small particle size and large surface area, this catalyst can provide better degradation results, compared to traditional processes. This work focuses on the elimination of pharmaceuticals present in different water matrices, considering the potential harm that these substances can cause in the environment. The mechanisms of pharmaceutical removal using Fe0 particles include reduction, adsorption, precipitation, and oxidation processes. Most studies have focused on oxidation processes in the presence of Fe0 and radicals derived from oxidants such as hydrogen peroxide (H2O2), ozone (O3), peroxysulfate (SO52−), peroxodisulfate (S2O82−), and oxygen (O2). Most of the results have shown that high percentages of pharmaceuticals can be removed, degraded, and mineralized. The mechanisms of oxidation and the parameters that influence the degradation of pharmaceuticals, as well as the possible degradation pathways, are discussed here. This review provides information on trends of different processes that use Fe0, considering aspects such as particle size, type of matrix, the pharmaceuticals studied, and the results obtained that can improve understanding of new advances in the field of advanced oxidation processes (AOPs) for the degradation and elimination of pharmaceuticals.
中文翻译:

使用零价铁(Fe 0)降解不同水基质中存在的药物的当前趋势
最近提出了零价铁(Fe 0)作为药物降解的潜在候选物,因为Fe 0可以释放出溶解的铁,活化分子氧,并与氧化剂发生反应。另外,由于其较小的粒径和较大的表面积,与传统方法相比,该催化剂可提供更好的降解结果。考虑到这些物质可能对环境造成的潜在危害,这项工作着重于消除存在于不同水基质中的药物。使用Fe 0颗粒去除药物的机理包括还原,吸附,沉淀和氧化过程。大多数研究都集中在铁存在下的氧化过程0从氧化剂如过氧化氢(H衍生和自由基2 ö 2),臭氧(O 3),peroxysulfate(SO 5 2- ),过二硫酸盐(š 2 ö 8 2- ),和氧(O 2)。大多数结果表明,高百分比的药物可以被去除,降解和矿化。这里讨论了氧化机理和影响药物降解的参数,以及可能的降解途径。这篇综述提供了有关使用Fe 0的不同过程的趋势的信息。考虑到诸如粒度,基质类型,研究的药物以及获得的结果等方面,可以更好地理解用于降解和消除药物的高级氧化工艺(AOP)领域的新进展。



























 京公网安备 11010802027423号
京公网安备 11010802027423号