当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
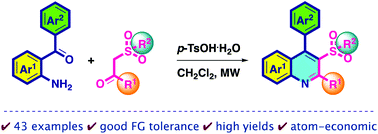
p-TsOH-mediated synthesis of substituted 2,4-diaryl-3-sulfonylquinolines from functionalized 2-aminobenzophenones and aromatic β-ketosulfones under microwave irradiation.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02445j Chieh-Kai Chan,Chien-Yu Lai,Wei-Chih Lo,Yu-Ting Cheng,Meng-Yang Chang,Cheng-Chung Wang
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-12-17 , DOI: 10.1039/c9ob02445j Chieh-Kai Chan,Chien-Yu Lai,Wei-Chih Lo,Yu-Ting Cheng,Meng-Yang Chang,Cheng-Chung Wang
|
This study describes an efficient protocol for the preparation of substituted 2,4-diaryl-3-sulfonylquinolines from functionalized 2-aminobenzophenones and aromatic β-ketosulfones by using p-toluenesulfonic acid monohydrate under microwave irradiation. In this atom-economical synthetic route, a series of pharmaceutically active 3-arylsulfonylquinolines with good functional group tolerance are prepared in good to excellent yields. Some structures are confirmed by single-crystal X-ray diffraction analysis.
中文翻译:

在微波辐射下,由功能化的2-氨基二苯甲酮和芳香族β-酮砜通过p-TsOH介导的取代的2,4-二芳基-3-磺酰基喹啉的合成。
这项研究描述了在微波辐射下使用对甲苯磺酸一水合物从官能化的2-氨基二苯甲酮和芳香族β-酮砜制备取代的2,4-二芳基-3-磺酰基喹啉的有效方案。在这种原子经济的合成途径中,以良好的至优异的产率制备了具有良好的官能团耐受性的一系列药物活性的3-芳基磺酰基喹啉。一些结构通过单晶X射线衍射分析确认。
更新日期:2020-01-15
中文翻译:

在微波辐射下,由功能化的2-氨基二苯甲酮和芳香族β-酮砜通过p-TsOH介导的取代的2,4-二芳基-3-磺酰基喹啉的合成。
这项研究描述了在微波辐射下使用对甲苯磺酸一水合物从官能化的2-氨基二苯甲酮和芳香族β-酮砜制备取代的2,4-二芳基-3-磺酰基喹啉的有效方案。在这种原子经济的合成途径中,以良好的至优异的产率制备了具有良好的官能团耐受性的一系列药物活性的3-芳基磺酰基喹啉。一些结构通过单晶X射线衍射分析确认。



























 京公网安备 11010802027423号
京公网安备 11010802027423号