当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The catalytic mechanism of the Au@TiO2−x/ZnO catalyst towards a low-temperature water-gas shift reaction
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019/12/11 , DOI: 10.1039/c9cy02077b Ning Liu 1, 2, 3, 4 , Pan Yin 1, 2, 3, 4 , Ming Xu 4, 5, 6, 7 , Yusen Yang 1, 2, 3, 4 , Shaomin Zhang 1, 2, 3, 4 , Junbo Zhang 1, 2, 3, 4 , Xiaoyu Meng 1, 2, 3, 4 , Jian Zhang 1, 2, 3, 4 , Jun Yu 1, 2, 3, 4 , Yi Man 4, 8, 9, 10 , Xin Zhang 1, 2, 3, 4 , Min Wei 1, 2, 3, 4
Catalysis Science & Technology ( IF 5 ) Pub Date : 2019/12/11 , DOI: 10.1039/c9cy02077b Ning Liu 1, 2, 3, 4 , Pan Yin 1, 2, 3, 4 , Ming Xu 4, 5, 6, 7 , Yusen Yang 1, 2, 3, 4 , Shaomin Zhang 1, 2, 3, 4 , Junbo Zhang 1, 2, 3, 4 , Xiaoyu Meng 1, 2, 3, 4 , Jian Zhang 1, 2, 3, 4 , Jun Yu 1, 2, 3, 4 , Yi Man 4, 8, 9, 10 , Xin Zhang 1, 2, 3, 4 , Min Wei 1, 2, 3, 4
Affiliation
|
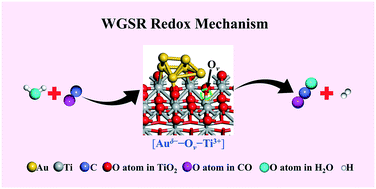
The mechanism of a low-temperature water-gas shift reaction (LT-WGSR) over supported gold catalysts has long been disputed, with the two prevailing ones being redox and associative mechanisms, due to the rather limited direct and reliable evidence for the actual reaction route. Herein, a typical supported gold-based catalyst (Au@TiO2−x/ZnO) was obtained via a structural topotactic transformation from hydrotalcite precursors, exhibiting an extremely high catalytic performance towards LT-WGSR. A series of experimental measurements involving in situ/operando DRIFTS, in situ Pulse-MASS and in situ/operando EXAFS as well as density functional theory (DFT) calculations were carried out to monitor the dynamic evolution of active sites, detect reaction intermediates and identify the reaction route. A new redox reaction route derived from the interfacial synergistic catalysis was verified: one H2O molecule undergoes dissociation at an Auδ−–Ov–Ti3+ (Ov: oxygen vacancy) interfacial active site, accompanied with the production of one H2 molecule and the oxidation of the interfacial structure to Au0–Or–Ti4+ (Or: the interfacial reactive oxygen species). Subsequently, one chemisorbed CO molecule captures Or to produce CO2 with the recovery of Auδ−–Ov–Ti3+. The present understanding and identification of the reaction mechanism of LT-WGSR would offer inspiration for further material exploration and catalyst design in gold-based heterogeneous catalysis.
中文翻译:

Au @ TiO2-x / ZnO催化剂对低温水煤气变换反应的催化机理
负载型金催化剂上的低温水煤气变换反应(LT-WGSR)的机理长期以来一直存在争议,主要的两种是氧化还原和缔合机理,因为对于实际反应而言,直接和可靠的证据相当有限路线。在此,通过水滑石前体的结构电位转变获得了典型的负载型金基催化剂(Au @ TiO 2- x / ZnO),表现出对LT-WGSR的极高的催化性能。一系列实验测量,包括原位/手术DRIFTS,原位Pulse-MASS和原位/手术进行了EXAFS以及密度泛函理论(DFT)的计算,以监测活性位点的动态演变,检测反应中间体并确定反应路线。验证了一种由界面协同催化作用产生的新的氧化还原反应途径:一个H 2 O分子在Auδ-- O v -Ti 3+(O v:氧空位)界面活性部位发生离解,并伴随一个H 2分子和界面结构被氧化为Au 0 –O r –Ti 4+(O r:界面活性氧种类)。随后,一个化学吸附的CO分子捕获了O r并产生了CO 2,并回收了Auδ-- O v -Ti 3+。目前对LT-WGSR反应机理的理解和鉴定将为进一步的金基多相催化材料探索和催化剂设计提供启示。
更新日期:2020-02-13
中文翻译:

Au @ TiO2-x / ZnO催化剂对低温水煤气变换反应的催化机理
负载型金催化剂上的低温水煤气变换反应(LT-WGSR)的机理长期以来一直存在争议,主要的两种是氧化还原和缔合机理,因为对于实际反应而言,直接和可靠的证据相当有限路线。在此,通过水滑石前体的结构电位转变获得了典型的负载型金基催化剂(Au @ TiO 2- x / ZnO),表现出对LT-WGSR的极高的催化性能。一系列实验测量,包括原位/手术DRIFTS,原位Pulse-MASS和原位/手术进行了EXAFS以及密度泛函理论(DFT)的计算,以监测活性位点的动态演变,检测反应中间体并确定反应路线。验证了一种由界面协同催化作用产生的新的氧化还原反应途径:一个H 2 O分子在Auδ-- O v -Ti 3+(O v:氧空位)界面活性部位发生离解,并伴随一个H 2分子和界面结构被氧化为Au 0 –O r –Ti 4+(O r:界面活性氧种类)。随后,一个化学吸附的CO分子捕获了O r并产生了CO 2,并回收了Auδ-- O v -Ti 3+。目前对LT-WGSR反应机理的理解和鉴定将为进一步的金基多相催化材料探索和催化剂设计提供启示。


























 京公网安备 11010802027423号
京公网安备 11010802027423号