当前位置:
X-MOL 学术
›
Faraday Discuss.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hydrogenation of substituted nitroaromatics on non-noble metal catalysts: mechanistic insights to improve selectivity
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9fd00126c Reisel Millán 1, 2, 3, 4, 5 , Mercedes Boronat 1, 2, 3, 4, 5
Faraday Discussions ( IF 3.4 ) Pub Date : 2019-12-11 , DOI: 10.1039/c9fd00126c Reisel Millán 1, 2, 3, 4, 5 , Mercedes Boronat 1, 2, 3, 4, 5
Affiliation
|
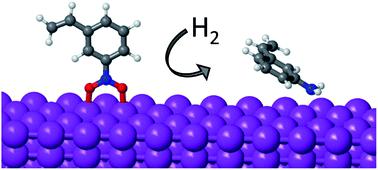
The mechanism of nitrobenzene hydrogenation on non-noble metals such as Ni is different from that previously reported for noble metals like Pt. The newly proposed pathway involves the initial dissociation of the two N–O bonds of nitrobenzene (Ph-NO2 → Ph-NO → Ph-N), leading to partial oxidation of the catalyst surface, followed by two successive hydrogenation steps (Ph-N → Ph-NH → Ph-NH2) that finally produce the functionalized aniline. Due to the oxophilic nature of non-noble metals like Ni, Co or Cu, the hydrogenation of the Ph-N intermediate and the removal of O in the form of water become the most energy demanding steps of the process. The strength of the interaction of O, H and N with different metals, and the preferential mode of adsorption of nitroarenes on clean and partially oxidized systems obtained from DFT calculations, are now used to propose an efficient non-noble metal catalyst that optimizes activity and selectivity.
中文翻译:

非贵金属催化剂上取代硝基芳香烃的加氢:提高选择性的机理见解
硝基苯在非贵金属(例如Ni)上的氢化机理与先前报道的在贵金属(例如Pt)上的氢化机理不同。新提出的途径涉及硝基苯的两个N–O键(Ph-NO 2 →Ph-NO→Ph-N)的初始解离,导致催化剂表面的部分氧化,然后是两个连续的加氢步骤(Ph- N→Ph-NH→Ph-NH 2),最终生成功能化的苯胺。由于非贵金属如Ni,Co或Cu的亲氧性质,Ph-N中间体的氢化和水形式的O的去除成为该过程中最耗能的步骤。O,H和N与不同金属的相互作用强度以及硝基芳烃在通过DFT计算获得的清洁和部分氧化的系统上的优先吸附模式,现在被用于提出一种有效的非贵金属催化剂,该催化剂可优化活性和选择性。
更新日期:2019-12-11
中文翻译:

非贵金属催化剂上取代硝基芳香烃的加氢:提高选择性的机理见解
硝基苯在非贵金属(例如Ni)上的氢化机理与先前报道的在贵金属(例如Pt)上的氢化机理不同。新提出的途径涉及硝基苯的两个N–O键(Ph-NO 2 →Ph-NO→Ph-N)的初始解离,导致催化剂表面的部分氧化,然后是两个连续的加氢步骤(Ph- N→Ph-NH→Ph-NH 2),最终生成功能化的苯胺。由于非贵金属如Ni,Co或Cu的亲氧性质,Ph-N中间体的氢化和水形式的O的去除成为该过程中最耗能的步骤。O,H和N与不同金属的相互作用强度以及硝基芳烃在通过DFT计算获得的清洁和部分氧化的系统上的优先吸附模式,现在被用于提出一种有效的非贵金属催化剂,该催化剂可优化活性和选择性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号