当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming.
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41416-019-0658-4 Ze Zhang 1 , Tian-En Li 1 , Mo Chen 1 , Da Xu 1 , Ying Zhu 1 , Bei-Yuan Hu 1 , Zhi-Fei Lin 1 , Jun-Jie Pan 1 , Xuan Wang 1 , Chao Wu 1 , Yan Zheng 1 , Lu Lu 1 , Hu-Liang Jia 1 , Song Gao 2 , Qiong-Zhu Dong 1, 3 , Lun-Xiu Qin 1, 3
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41416-019-0658-4 Ze Zhang 1 , Tian-En Li 1 , Mo Chen 1 , Da Xu 1 , Ying Zhu 1 , Bei-Yuan Hu 1 , Zhi-Fei Lin 1 , Jun-Jie Pan 1 , Xuan Wang 1 , Chao Wu 1 , Yan Zheng 1 , Lu Lu 1 , Hu-Liang Jia 1 , Song Gao 2 , Qiong-Zhu Dong 1, 3 , Lun-Xiu Qin 1, 3
Affiliation
|
BACKGROUND
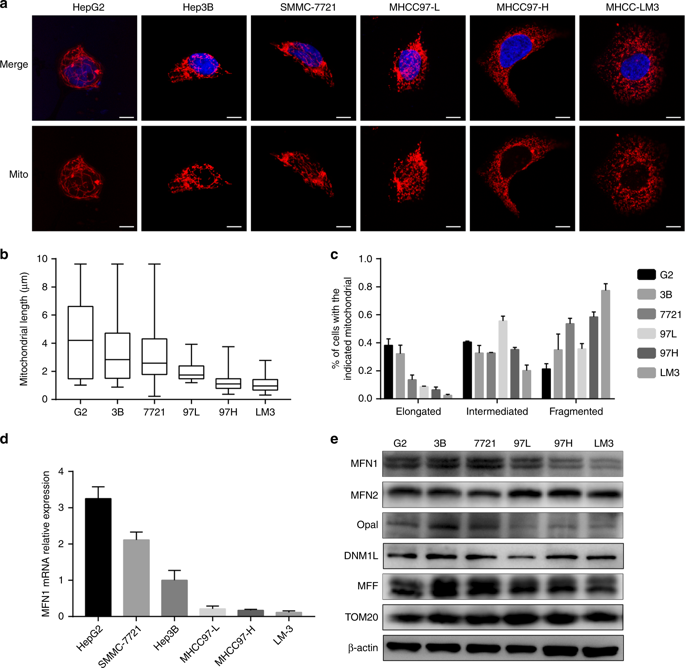
Mitochondrial dynamics plays an important role in tumour progression. However, how these dynamics integrate tumour metabolism in hepatocellular carcinoma (HCC) metastasis is still unclear.
METHODS
The mitochondrial fusion protein mitofusin-1 (MFN1) expression and its prognostic value are detected in HCC. The effects and underlying mechanisms of MFN1 on HCC metastasis and metabolic reprogramming are analysed both in vitro and in vivo.
RESULTS
Mitochondrial dynamics, represented by constant fission and fusion, are found to be associated with HCC metastasis. High metastatic HCC displays excessive mitochondrial fission. Among genes involved in mitochondrial dynamics, MFN1 is identified as a leading downregulated candidate that is closely associated with HCC metastasis and poor prognosis. While promoting mitochondrial fusion, MFN1 inhibits cell proliferation, invasion and migration capacity both in vitro and in vivo. Mechanistically, disruption of mitochondrial dynamics by depletion of MFN1 triggers the epithelial-to-mesenchymal transition (EMT) of HCC. Moreover, MFN1 modulates HCC metastasis by metabolic shift from aerobic glycolysis to oxidative phosphorylation. Treatment with glycolytic inhibitor 2-Deoxy-D-glucose (2-DG) significantly suppresses the effects induced by depletion of MFN1.
CONCLUSIONS
Our results reveal a critical involvement of mitochondrial dynamics in HCC metastasis via modulating glucose metabolic reprogramming. MFN1 may serve as a novel potential therapeutic target for HCC.
中文翻译:

MFN1 依赖性线粒体动力学改变通过葡萄糖代谢重编程驱动肝细胞癌转移。
背景线粒体动力学在肿瘤进展中起重要作用。然而,这些动力学如何整合肝细胞癌 (HCC) 转移中的肿瘤代谢仍不清楚。方法检测HCC中线粒体融合蛋白mitofusin-1(MFN1)的表达及其预后价值。在体外和体内分析了 MFN1 对 HCC 转移和代谢重编程的影响和潜在机制。结果发现以恒定裂变和融合为代表的线粒体动力学与HCC转移有关。高转移性 HCC 表现出过度的线粒体裂变。在涉及线粒体动力学的基因中,MFN1 被确定为与 HCC 转移和预后不良密切相关的主要下调候选基因。在促进线粒体融合的同时,MFN1 在体外和体内均抑制细胞增殖、侵袭和迁移能力。从机制上讲,MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。通过 MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。通过 MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。
更新日期:2019-12-11
中文翻译:

MFN1 依赖性线粒体动力学改变通过葡萄糖代谢重编程驱动肝细胞癌转移。
背景线粒体动力学在肿瘤进展中起重要作用。然而,这些动力学如何整合肝细胞癌 (HCC) 转移中的肿瘤代谢仍不清楚。方法检测HCC中线粒体融合蛋白mitofusin-1(MFN1)的表达及其预后价值。在体外和体内分析了 MFN1 对 HCC 转移和代谢重编程的影响和潜在机制。结果发现以恒定裂变和融合为代表的线粒体动力学与HCC转移有关。高转移性 HCC 表现出过度的线粒体裂变。在涉及线粒体动力学的基因中,MFN1 被确定为与 HCC 转移和预后不良密切相关的主要下调候选基因。在促进线粒体融合的同时,MFN1 在体外和体内均抑制细胞增殖、侵袭和迁移能力。从机制上讲,MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。通过 MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。通过 MFN1 的消耗破坏线粒体动力学会触发 HCC 的上皮间质转化 (EMT)。此外,MFN1 通过从有氧糖酵解到氧化磷酸化的代谢转变来调节 HCC 转移。用糖酵解抑制剂 2-脱氧-D-葡萄糖 (2-DG) 治疗可显着抑制 MFN1 消耗引起的影响。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。结论 我们的结果揭示了线粒体动力学通过调节葡萄糖代谢重编程在 HCC 转移中的关键作用。MFN1 可作为 HCC 的一个新的潜在治疗靶点。



























 京公网安备 11010802027423号
京公网安备 11010802027423号