当前位置:
X-MOL 学术
›
Br. J. Cancer
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Cutting off the fuel supply to calcium pumps in pancreatic cancer cells: role of pyruvate kinase-M2 (PKM2).
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41416-019-0675-3 Andrew D James 1, 2 , Daniel A Richardson 1 , In-Whan Oh 1 , Pishyaporn Sritangos 1 , Thomas Attard 1 , Lisa Barrett 1 , Jason I E Bruce 1
British Journal of Cancer ( IF 8.8 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41416-019-0675-3 Andrew D James 1, 2 , Daniel A Richardson 1 , In-Whan Oh 1 , Pishyaporn Sritangos 1 , Thomas Attard 1 , Lisa Barrett 1 , Jason I E Bruce 1
Affiliation
|
BACKGROUND
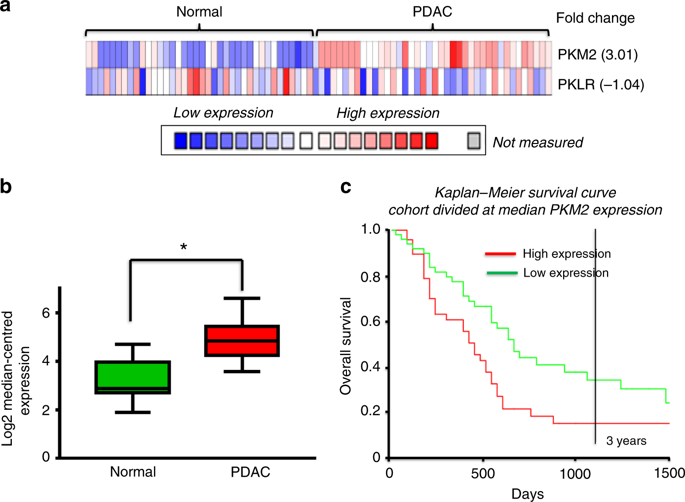
Pancreatic ductal adenocarcinoma (PDAC) has poor survival and treatment options. PDAC cells shift their metabolism towards glycolysis, which fuels the plasma membrane calcium pump (PMCA), thereby preventing Ca2+-dependent cell death. The ATP-generating pyruvate kinase-M2 (PKM2) is oncogenic and overexpressed in PDAC. This study investigated the PKM2-derived ATP supply to the PMCA as a potential therapeutic locus.
METHODS
PDAC cell growth, migration and death were assessed by using sulforhodamine-B/tetrazolium-based assays, gap closure assay and poly-ADP ribose polymerase (PARP1) cleavage, respectively. Cellular ATP and metabolism were assessed using luciferase/fluorescent-based assays and the Seahorse XFe96 analyzer, respectively. Cell surface biotinylation identified membrane-associated proteins. Fura-2 imaging was used to assess cytosolic Ca2+ overload and in situ Ca2+ clearance. PKM2 knockdown was achieved using siRNA.
RESULTS
The PKM2 inhibitor (shikonin) reduced PDAC cell proliferation, cell migration and induced cell death. This was due to inhibition of glycolysis, ATP depletion, inhibition of PMCA and cytotoxic Ca2+ overload. PKM2 associates with plasma membrane proteins providing a privileged ATP supply to the PMCA. PKM2 knockdown reduced PMCA activity and reduced the sensitivity of shikonin-induced cell death.
CONCLUSIONS
Cutting off the PKM2-derived ATP supply to the PMCA represents a novel therapeutic strategy for the treatment of PDAC.
中文翻译:

切断胰腺癌细胞钙泵的燃料供应:丙酮酸激酶-M2(PKM2)的作用。
背景胰腺导管腺癌 (PDAC) 的存活率和治疗选择都很差。PDAC 细胞将其新陈代谢转向糖酵解,从而为质膜钙泵 (PMCA) 提供燃料,从而防止 Ca2+ 依赖性细胞死亡。产生 ATP 的丙酮酸激酶-M2 (PKM2) 在 PDAC 中具有致癌性和过表达。本研究调查了 PMCA 的 PKM2 衍生的 ATP 供应作为潜在的治疗位点。方法 PDAC 细胞生长、迁移和死亡分别通过使用基于磺基罗丹明-B/四唑的测定、间隙闭合测定和聚-ADP 核糖聚合酶 (PARP1) 切割进行评估。分别使用基于荧光素酶/荧光的测定和 Seahorse XFe96 分析仪评估细胞 ATP 和代谢。细胞表面生物素化鉴定了膜相关蛋白。Fura-2 成像用于评估细胞溶质 Ca2+ 超载和原位 Ca2+ 清除。使用 siRNA 实现了 PKM2 敲低。结果 PKM2 抑制剂(紫草素)降低了 PDAC 细胞增殖、细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。
更新日期:2019-12-11
中文翻译:

切断胰腺癌细胞钙泵的燃料供应:丙酮酸激酶-M2(PKM2)的作用。
背景胰腺导管腺癌 (PDAC) 的存活率和治疗选择都很差。PDAC 细胞将其新陈代谢转向糖酵解,从而为质膜钙泵 (PMCA) 提供燃料,从而防止 Ca2+ 依赖性细胞死亡。产生 ATP 的丙酮酸激酶-M2 (PKM2) 在 PDAC 中具有致癌性和过表达。本研究调查了 PMCA 的 PKM2 衍生的 ATP 供应作为潜在的治疗位点。方法 PDAC 细胞生长、迁移和死亡分别通过使用基于磺基罗丹明-B/四唑的测定、间隙闭合测定和聚-ADP 核糖聚合酶 (PARP1) 切割进行评估。分别使用基于荧光素酶/荧光的测定和 Seahorse XFe96 分析仪评估细胞 ATP 和代谢。细胞表面生物素化鉴定了膜相关蛋白。Fura-2 成像用于评估细胞溶质 Ca2+ 超载和原位 Ca2+ 清除。使用 siRNA 实现了 PKM2 敲低。结果 PKM2 抑制剂(紫草素)降低了 PDAC 细胞增殖、细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。细胞迁移和诱导细胞死亡。这是由于抑制了糖酵解、ATP 消耗、抑制了 PMCA 和细胞毒性 Ca2+ 超载。PKM2 与为 PMCA 提供特权 ATP 供应的质膜蛋白结合。PKM2 敲低降低了 PMCA 活性并降低了紫草素诱导的细胞死亡的敏感性。结论 切断对 PMCA 的 PKM2 衍生的 ATP 供应代表了一种治疗 PDAC 的新治疗策略。


























 京公网安备 11010802027423号
京公网安备 11010802027423号