当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of 2,2,2‐Trifluoroethyl Oxazoles, Oxazolines and Furans via Alkyne Oxytrifluoromethylation
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-07 , DOI: 10.1002/adsc.201901405 Jia‐Jia Dong 1 , Song‐Lin Zhang 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2020-01-07 , DOI: 10.1002/adsc.201901405 Jia‐Jia Dong 1 , Song‐Lin Zhang 1
Affiliation
|
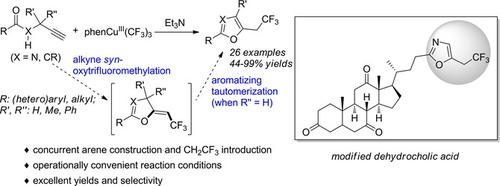
This study reports an oxytrifluoromethylation method for construction of oxazoles and furans motif and the concurrent incorporation of a 2,2,2‐trifluoroethyl group at the aromatic C5‐position. High‐valent copper(III) trifluoromethyl compounds are crucial to this reaction that induces oxy‐trifluoromethylation of alkynes with a pendant amide/enol group functioning as the oxygen‐nucleophile. A wide substrate scope is demonstrated with high efficiency and with broad functional group tolerance. Late‐stage functionalization of dehydrocholic acid, a complex drug compound, is accomplished to show the potential of this method for practical drug design.
中文翻译:

通过炔基氧化三氟甲基化合成2,2,2-三氟乙基恶唑,恶唑啉和呋喃
这项研究报告了一种氧三氟甲基化方法,用于构建恶唑和呋喃基序,并在芳族C5位上同时引入2,2,2-三氟乙基。高价的三氟甲基铜(III)对于此反应至关重要,该反应可诱导具有侧链酰胺/烯醇基团的炔烃的氧-三氟甲基化,并起氧亲核试剂的作用。高效率和宽泛的官能团耐受性证明了广泛的底物范围。完成了脱氢胆酸(一种复杂的药物化合物)的后期功能化,以显示该方法在实际药物设计中的潜力。
更新日期:2020-01-07
中文翻译:

通过炔基氧化三氟甲基化合成2,2,2-三氟乙基恶唑,恶唑啉和呋喃
这项研究报告了一种氧三氟甲基化方法,用于构建恶唑和呋喃基序,并在芳族C5位上同时引入2,2,2-三氟乙基。高价的三氟甲基铜(III)对于此反应至关重要,该反应可诱导具有侧链酰胺/烯醇基团的炔烃的氧-三氟甲基化,并起氧亲核试剂的作用。高效率和宽泛的官能团耐受性证明了广泛的底物范围。完成了脱氢胆酸(一种复杂的药物化合物)的后期功能化,以显示该方法在实际药物设计中的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号