当前位置:
X-MOL 学术
›
Mol. Omics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regulatory network analysis of Paneth cell and goblet cell enriched gut organoids using transcriptomics approaches.
Molecular Omics ( IF 2.9 ) Pub Date : 2020-02-17 , DOI: 10.1039/c9mo00130a A Treveil 1 , P Sudhakar , Z J Matthews , T Wrzesiński , E J Jones , J Brooks , M Ölbei , I Hautefort , L J Hall , S R Carding , U Mayer , P P Powell , T Wileman , F Di Palma , W Haerty , T Korcsmáros
Molecular Omics ( IF 2.9 ) Pub Date : 2020-02-17 , DOI: 10.1039/c9mo00130a A Treveil 1 , P Sudhakar , Z J Matthews , T Wrzesiński , E J Jones , J Brooks , M Ölbei , I Hautefort , L J Hall , S R Carding , U Mayer , P P Powell , T Wileman , F Di Palma , W Haerty , T Korcsmáros
Affiliation
|
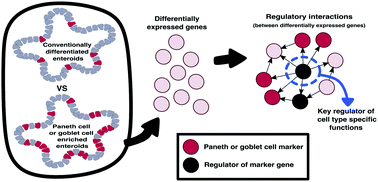
The epithelial lining of the small intestine consists of multiple cell types, including Paneth cells and goblet cells, that work in cohort to maintain gut health. 3D in vitro cultures of human primary epithelial cells, called organoids, have become a key model to study the functions of Paneth cells and goblet cells in normal and diseased conditions. Advances in these models include the ability to skew differentiation to particular lineages, providing a useful tool to study cell type specific function/dysfunction in the context of the epithelium. Here, we use comprehensive profiling of mRNA, microRNA and long non-coding RNA expression to confirm that Paneth cell and goblet cell enrichment of murine small intestinal organoids (enteroids) establishes a physiologically accurate model. We employ network analysis to infer the regulatory landscape altered by skewing differentiation, and using knowledge of cell type specific markers, we predict key regulators of cell type specific functions: Cebpa, Jun, Nr1d1 and Rxra specific to Paneth cells, Gfi1b and Myc specific for goblet cells and Ets1, Nr3c1 and Vdr shared between them. Links identified between these regulators and cellular phenotypes of inflammatory bowel disease (IBD) suggest that global regulatory rewiring during or after differentiation of Paneth cells and goblet cells could contribute to IBD aetiology. Future application of cell type enriched enteroids combined with the presented computational workflow can be used to disentangle multifactorial mechanisms of these cell types and propose regulators whose pharmacological targeting could be advantageous in treating IBD patients with Crohn's disease or ulcerative colitis.
中文翻译:

使用转录组学方法对潘氏细胞和杯状细胞富集的肠道类器官进行调控网络分析。
小肠的上皮内壁由多种细胞类型组成,包括潘氏细胞和杯状细胞,它们协同工作以维持肠道健康。人类原代上皮细胞(称为类器官)的 3D 体外培养物已成为研究潘氏细胞和杯状细胞在正常和患病条件下功能的关键模型。这些模型的进步包括将分化偏向特定谱系的能力,为研究上皮细胞类型特定功能/功能障碍提供了有用的工具。在这里,我们使用 mRNA、microRNA 和长链非编码 RNA 表达的综合分析来确认小鼠小肠类器官 (enteroids) 的潘氏细胞和杯状细胞富集建立了生理上准确的模型。我们采用网络分析来推断因倾斜分化而改变的调控格局,并利用细胞类型特异性标记的知识,我们预测细胞类型特异性功能的关键调控因子:Cebpa、Jun、Nr1d1 和 Rxra 特定于潘氏细胞,Gfi1b 和 Myc 特定于杯状细胞和 Ets1、Nr3c1 和 Vdr 在它们之间共享。这些监管机构与炎症性肠病 (IBD) 的细胞表型之间确定的联系表明,潘氏细胞和杯状细胞分化期间或之后的全球监管重新布线可能有助于 IBD 病因学。
更新日期:2020-02-18
中文翻译:

使用转录组学方法对潘氏细胞和杯状细胞富集的肠道类器官进行调控网络分析。
小肠的上皮内壁由多种细胞类型组成,包括潘氏细胞和杯状细胞,它们协同工作以维持肠道健康。人类原代上皮细胞(称为类器官)的 3D 体外培养物已成为研究潘氏细胞和杯状细胞在正常和患病条件下功能的关键模型。这些模型的进步包括将分化偏向特定谱系的能力,为研究上皮细胞类型特定功能/功能障碍提供了有用的工具。在这里,我们使用 mRNA、microRNA 和长链非编码 RNA 表达的综合分析来确认小鼠小肠类器官 (enteroids) 的潘氏细胞和杯状细胞富集建立了生理上准确的模型。我们采用网络分析来推断因倾斜分化而改变的调控格局,并利用细胞类型特异性标记的知识,我们预测细胞类型特异性功能的关键调控因子:Cebpa、Jun、Nr1d1 和 Rxra 特定于潘氏细胞,Gfi1b 和 Myc 特定于杯状细胞和 Ets1、Nr3c1 和 Vdr 在它们之间共享。这些监管机构与炎症性肠病 (IBD) 的细胞表型之间确定的联系表明,潘氏细胞和杯状细胞分化期间或之后的全球监管重新布线可能有助于 IBD 病因学。


























 京公网安备 11010802027423号
京公网安备 11010802027423号