当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and Pharmacological Characterization of Conformationally Restricted Retigabine Analogues as Novel Neuronal Kv7 Channel Activators.
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jmedchem.9b00796 Carmine Ostacolo 1 , Francesco Miceli 2 , Veronica Di Sarno 3 , Piera Nappi 2 , Nunzio Iraci 3 , Maria Virginia Soldovieri 4 , Tania Ciaglia 3 , Paolo Ambrosino 5 , Vincenzo Vestuto 3 , Anna Lauritano 2 , Simona Musella 6 , Giacomo Pepe 3 , Manuela Giovanna Basilicata 3 , Michele Manfra 7 , Diego Romano Perinelli 8 , Ettore Novellino 1 , Alessia Bertamino 3 , Isabel M Gomez-Monterrey 1 , Pietro Campiglia 3 , Maurizio Taglialatela 2
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2019-12-24 , DOI: 10.1021/acs.jmedchem.9b00796 Carmine Ostacolo 1 , Francesco Miceli 2 , Veronica Di Sarno 3 , Piera Nappi 2 , Nunzio Iraci 3 , Maria Virginia Soldovieri 4 , Tania Ciaglia 3 , Paolo Ambrosino 5 , Vincenzo Vestuto 3 , Anna Lauritano 2 , Simona Musella 6 , Giacomo Pepe 3 , Manuela Giovanna Basilicata 3 , Michele Manfra 7 , Diego Romano Perinelli 8 , Ettore Novellino 1 , Alessia Bertamino 3 , Isabel M Gomez-Monterrey 1 , Pietro Campiglia 3 , Maurizio Taglialatela 2
Affiliation
|
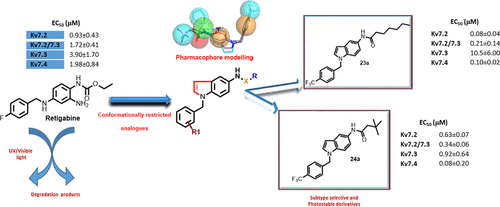
Kv7 K+ channels represent attractive pharmacological targets for the treatment of different neurological disorders, including epilepsy. In this paper, 42 conformationally restricted analogues of the prototypical Kv7 activator retigabine have been synthesized and tested by electrophysiological patch-clamp experiments as Kv7 agonists. When compared to retigabine (0.93 ± 0.43 μM), the EC50s for Kv7.2 current enhancements by compound 23a (0.08 ± 0.04 μM) were lower, whereas no change in potency was observed for 24a (0.63 ± 0.07 μM). In addition, compared to retigabine, 23a and 24a showed also higher potency in activating heteromeric Kv7.2/Kv7.3 and homomeric Kv7.4 channels. Molecular modeling studies provided new insights into the chemical features required for optimal interaction at the binding site. Stability studies evidenced improved chemical stability of 23a and 24a in comparison with retigabine. Overall, the present results highlight that the N5-alkylamidoindole moiety provides a suitable pharmacophoric scaffold for the design of chemically stable, highly potent and selective Kv7 agonists.
中文翻译:

构象受限的瑞替加滨类似物的合成和药理学表征,作为新型神经元Kv7通道激活剂。
Kv7 K +通道代表了用于治疗包括癫痫在内的各种神经系统疾病的有吸引力的药理靶标。在本文中,已合成了42个原型Kv7激活剂瑞替加滨构象受限的类似物,并通过电生理膜片钳实验作为Kv7激动剂进行了测试。与瑞替加滨(0.93±0.43μM)相比,化合物23a增强Kv7.2电流的EC50(0.08±0.04μM)较低,而24a(0.63±0.07μM)的效力未见变化。此外,与瑞替加滨相比,23a和24a在激活异聚Kv7.2 / Kv7.3和同聚Kv7.4通道方面也显示出更高的效力。分子模型研究为结合位点的最佳相互作用所需的化学特征提供了新的见解。稳定性研究表明,与瑞替加滨相比,化学稳定性提高了23a和24a。总体而言,本发明结果突出了N5-烷基酰胺基吲哚部分为设计化学稳定的,高效的和选择性的Kv7激动剂提供了合适的药效学支架。
更新日期:2019-12-25
中文翻译:

构象受限的瑞替加滨类似物的合成和药理学表征,作为新型神经元Kv7通道激活剂。
Kv7 K +通道代表了用于治疗包括癫痫在内的各种神经系统疾病的有吸引力的药理靶标。在本文中,已合成了42个原型Kv7激活剂瑞替加滨构象受限的类似物,并通过电生理膜片钳实验作为Kv7激动剂进行了测试。与瑞替加滨(0.93±0.43μM)相比,化合物23a增强Kv7.2电流的EC50(0.08±0.04μM)较低,而24a(0.63±0.07μM)的效力未见变化。此外,与瑞替加滨相比,23a和24a在激活异聚Kv7.2 / Kv7.3和同聚Kv7.4通道方面也显示出更高的效力。分子模型研究为结合位点的最佳相互作用所需的化学特征提供了新的见解。稳定性研究表明,与瑞替加滨相比,化学稳定性提高了23a和24a。总体而言,本发明结果突出了N5-烷基酰胺基吲哚部分为设计化学稳定的,高效的和选择性的Kv7激动剂提供了合适的药效学支架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号