当前位置:
X-MOL 学术
›
npj Genom. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
FDA oversight of NSIGHT genomic research: the need for an integrated systems approach to regulation.
npj Genomic Medicine ( IF 5.3 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41525-019-0105-8 Laura V Milko 1 , Flavia Chen 2, 3 , Kee Chan 4 , Amy M Brower 5 , Pankaj B Agrawal 6, 7, 8 , Alan H Beggs 6, 8 , Jonathan S Berg 1 , Steven E Brenner 2, 9 , Ingrid A Holm 6, 8 , Barbara A Koenig 2, 3 , Richard B Parad 8, 10 , Cynthia M Powell 1, 11 , Stephen F Kingsmore 12
npj Genomic Medicine ( IF 5.3 ) Pub Date : 2019-12-10 , DOI: 10.1038/s41525-019-0105-8 Laura V Milko 1 , Flavia Chen 2, 3 , Kee Chan 4 , Amy M Brower 5 , Pankaj B Agrawal 6, 7, 8 , Alan H Beggs 6, 8 , Jonathan S Berg 1 , Steven E Brenner 2, 9 , Ingrid A Holm 6, 8 , Barbara A Koenig 2, 3 , Richard B Parad 8, 10 , Cynthia M Powell 1, 11 , Stephen F Kingsmore 12
Affiliation
|
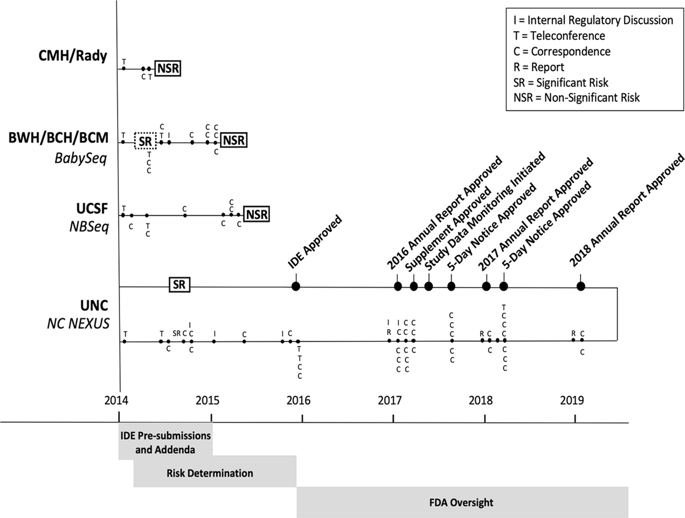
The National Institutes of Health (NIH) funded the Newborn Sequencing In Genomic medicine and public HealTh (NSIGHT) Consortium to investigate the implications, challenges, and opportunities associated with the possible use of genomic sequence information in the newborn period. Following announcement of the NSIGHT awardees in 2013, the Food and Drug Administration (FDA) contacted investigators and requested that pre-submissions to investigational device exemptions (IDE) be submitted for the use of genomic sequencing under Title 21 of the Code of Federal Regulations (21 CFR) part 812. IDE regulation permits clinical investigation of medical devices that have not been approved by the FDA. To our knowledge, this marked the first time the FDA determined that NIH-funded clinical genomic research projects are subject to IDE regulation. Here, we review the history of and rationale behind FDA oversight of clinical research and the NSIGHT Consortium's experiences in navigating the IDE process. Overall, NSIGHT investigators found that FDA's application of existing IDE regulations and medical device definitions aligned imprecisely with the aims of publicly funded exploratory clinical research protocols. IDE risk assessments by the FDA were similar to, but distinct from, protocol risk assessments conducted by local Institutional Review Boards (IRBs), and had the potential to reflect novel oversight of emerging genomic technologies. However, the pre-IDE and IDE process delayed the start of NSIGHT research studies by an average of 10 months, and significantly limited the scope of investigation in two of the four NIH approved projects. Based on the experience of the NSIGHT Consortium, we conclude that policies and practices governing the development and use of novel genomic technologies in clinical research urgently need clarification in order to mitigate potentially conflicting or redundant oversight by IRBs, NIH, FDA, and state authorities.
中文翻译:

FDA对NSIGHT基因组研究的监督:需要采用集成系统进行监管的方法。
美国国立卫生研究院(NIH)资助了基因组医学新生儿测序和公共健康(NSIGHT)联盟,以研究与新生儿时期可能使用基因组序列信息有关的影响,挑战和机会。在2013年宣布获得NSIGHT获奖者之后,美国食品药品监督管理局(FDA)与调查人员联系,并要求根据联邦法规第21篇第21条的规定,就使用基因组测序的技术提交预先提交的研究仪器豁免(IDE)。 21 CFR)第812部分。IDE法规允许对未经FDA批准的医疗器械进行临床研究。据我们所知,这标志着FDA首次确定NIH资助的临床基因组研究项目应受IDE监管。这里,我们回顾了FDA对临床研究的监督的历史和背后原因以及NSIGHT联盟在IDE流程中的经验。总体而言,NSIGHT研究人员发现,FDA对现有IDE法规和医疗设备定义的应用与公共资助的探索性临床研究协议的目标不完全一致。FDA进行的IDE风险评估与地方机构审查委员会(IRB)进行的协议风险评估相似但有区别,并且有可能反映对新兴基因组技术的新颖监督。但是,IDE和IDE之前的流程将NSIGHT研究的开始平均延迟了10个月,并且极大地限制了NIH批准的四个项目中的两个项目的研究范围。根据NSIGHT联盟的经验,
更新日期:2019-12-10
中文翻译:

FDA对NSIGHT基因组研究的监督:需要采用集成系统进行监管的方法。
美国国立卫生研究院(NIH)资助了基因组医学新生儿测序和公共健康(NSIGHT)联盟,以研究与新生儿时期可能使用基因组序列信息有关的影响,挑战和机会。在2013年宣布获得NSIGHT获奖者之后,美国食品药品监督管理局(FDA)与调查人员联系,并要求根据联邦法规第21篇第21条的规定,就使用基因组测序的技术提交预先提交的研究仪器豁免(IDE)。 21 CFR)第812部分。IDE法规允许对未经FDA批准的医疗器械进行临床研究。据我们所知,这标志着FDA首次确定NIH资助的临床基因组研究项目应受IDE监管。这里,我们回顾了FDA对临床研究的监督的历史和背后原因以及NSIGHT联盟在IDE流程中的经验。总体而言,NSIGHT研究人员发现,FDA对现有IDE法规和医疗设备定义的应用与公共资助的探索性临床研究协议的目标不完全一致。FDA进行的IDE风险评估与地方机构审查委员会(IRB)进行的协议风险评估相似但有区别,并且有可能反映对新兴基因组技术的新颖监督。但是,IDE和IDE之前的流程将NSIGHT研究的开始平均延迟了10个月,并且极大地限制了NIH批准的四个项目中的两个项目的研究范围。根据NSIGHT联盟的经验,


























 京公网安备 11010802027423号
京公网安备 11010802027423号