当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Organocatalytic Asymmetric Synthesis of Indole-Based Chiral Heterocycles: Strategies, Reactions, and Outreach.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.accounts.9b00549 Yu-Chen Zhang 1 , Fei Jiang 1 , Feng Shi 1
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-10 , DOI: 10.1021/acs.accounts.9b00549 Yu-Chen Zhang 1 , Fei Jiang 1 , Feng Shi 1
Affiliation
|
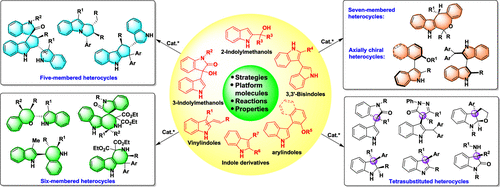
Indole-based chiral heterocycles constitute a class of important heterocyclic compounds that are found in numerous pharmaceuticals, functional materials, and chiral catalysts or ligands. Catalytic asymmetric synthesis, for which the 2001 Nobel Prize in Chemistry was awarded, has been demonstrated to be the most efficient method for accessing chiral compounds. Therefore, the catalytic asymmetric synthesis of indole-based chiral heterocycles has attracted great interest from the scientific community. However, the strategies toward this goal are rather limited, and great challenges remain in this field, such as metal contamination in the products, the limited number of platform molecules with versatile reactivity, and the limited number of catalytic asymmetric reactions that offer high step economy, atom economy, and excellent enantiocontrol. Therefore, novel strategies for the catalytic asymmetric synthesis of indole-based chiral heterocycles are urgently needed. To achieve this goal, our group has developed a series of unique strategies, such as designing and developing versatile platform molecules and their corresponding organocatalytic asymmetric reactions to access indole-based chiral heterocycles. In this Account, we describe our efforts to address the remaining challenges in this research field. Namely, we have designed and developed vinylindoles, indolylmethanols, arylindoles and indole derivatives as versatile platform molecules for the construction of indole-based chiral heterocyclic scaffolds with structural diversity and complexity. Based on the reactivities of these platform molecules, we have designed and accomplished a series of organocatalytic asymmetric cycloaddition, cyclization, addition and dearomatization reactions with a high step economy, atom economy and excellent enantiocontrol. Using these strategies, a wide range of indole-based chiral heterocycles, including five-membered to seven-membered heterocycles, axially chiral heterocycles and tetrasubstituted heterocycles, have been synthesized with high efficiency and excellent enantioselectivity. In addition, we have investigated the properties of some indole-based chiral heterocycles, including their bioactivities and catalytic activities, and showed that these chiral heterocycles have potent anticancer activities and promising catalytic activities in asymmetric catalysis. These results help elucidate the potential applications of indole-based chiral heterocycles in drug development and chiral catalysts. The organocatalytic asymmetric synthesis of indole-based chiral heterocycles has undoubtedly become and will continue to be a hot topic in the field of asymmetric catalysis and synthesis. Our efforts, summarized in this Account, will not only open a window for the future development of innovative strategies toward organocatalytic asymmetric synthesis of indole-based chiral heterocycles but also inspire chemists worldwide to confront the remaining challenges in this field and prompt further advances.
中文翻译:

基于吲哚的手性杂环的有机催化不对称合成:策略,反应和拓展。
基于吲哚的手性杂环构成了一类重要的杂环化合物,可在许多药物,功能材料和手性催化剂或配体中找到。事实证明,催化不对称合成是获得手性化合物最有效的方法,该方法获得了2001年诺贝尔化学奖。因此,基于吲哚的手性杂环的催化不对称合成引起了科学界的极大兴趣。但是,实现该目标的策略非常有限,并且在该领域中仍然存在巨大挑战,例如产品中的金属污染,具有多种反应性的平台分子数量有限以及提供高步经济性的催化不对称反应数量有限,原子经济和出色的对映控制。因此,迫切需要用于基于吲哚的手性杂环的催化不对称合成的新策略。为了实现此目标,我们小组开发了一系列独特的策略,例如设计和开发通用平台分子及其相应的有机催化不对称反应,以接近基于吲哚的手性杂环。在本报告中,我们描述了我们为解决该研究领域中剩余挑战而做出的努力。即,我们已经设计并开发了乙烯基吲哚,吲哚基甲醇,芳基吲哚和吲哚衍生物作为通用平台分子,用于构建具有结构多样性和复杂性的基于吲哚的手性杂环骨架。基于这些平台分子的反应性,我们设计并完成了一系列有机催化不对称环加成反应,具有高步骤经济性,原子经济性和出色的对映体控制能力的环化,加成和脱芳香化反应。使用这些策略,已经合成了多种基于吲哚的手性杂环,包括五元至七元杂环,轴向手性杂环和四取代杂环,它们具有很高的效率和出色的对映选择性。此外,我们已经研究了一些基于吲哚的手性杂环的性质,包括其生物活性和催化活性,并表明这些手性杂环在非对称催化中具有有效的抗癌活性和有希望的催化活性。这些结果有助于阐明基于吲哚的手性杂环在药物开发和手性催化剂中的潜在应用。吲哚基手性杂环的有机催化不对称合成无疑已经成为并将继续成为不对称催化和合成领域中的热门话题。在本报告中总结的我们的努力,不仅将为今后发展基于吲哚基手性杂环的有机催化不对称合成的创新策略打开一扇窗,而且还将激发全世界的化学家应对这一领域中的剩余挑战并推动进一步的发展。
更新日期:2019-12-11
中文翻译:

基于吲哚的手性杂环的有机催化不对称合成:策略,反应和拓展。
基于吲哚的手性杂环构成了一类重要的杂环化合物,可在许多药物,功能材料和手性催化剂或配体中找到。事实证明,催化不对称合成是获得手性化合物最有效的方法,该方法获得了2001年诺贝尔化学奖。因此,基于吲哚的手性杂环的催化不对称合成引起了科学界的极大兴趣。但是,实现该目标的策略非常有限,并且在该领域中仍然存在巨大挑战,例如产品中的金属污染,具有多种反应性的平台分子数量有限以及提供高步经济性的催化不对称反应数量有限,原子经济和出色的对映控制。因此,迫切需要用于基于吲哚的手性杂环的催化不对称合成的新策略。为了实现此目标,我们小组开发了一系列独特的策略,例如设计和开发通用平台分子及其相应的有机催化不对称反应,以接近基于吲哚的手性杂环。在本报告中,我们描述了我们为解决该研究领域中剩余挑战而做出的努力。即,我们已经设计并开发了乙烯基吲哚,吲哚基甲醇,芳基吲哚和吲哚衍生物作为通用平台分子,用于构建具有结构多样性和复杂性的基于吲哚的手性杂环骨架。基于这些平台分子的反应性,我们设计并完成了一系列有机催化不对称环加成反应,具有高步骤经济性,原子经济性和出色的对映体控制能力的环化,加成和脱芳香化反应。使用这些策略,已经合成了多种基于吲哚的手性杂环,包括五元至七元杂环,轴向手性杂环和四取代杂环,它们具有很高的效率和出色的对映选择性。此外,我们已经研究了一些基于吲哚的手性杂环的性质,包括其生物活性和催化活性,并表明这些手性杂环在非对称催化中具有有效的抗癌活性和有希望的催化活性。这些结果有助于阐明基于吲哚的手性杂环在药物开发和手性催化剂中的潜在应用。吲哚基手性杂环的有机催化不对称合成无疑已经成为并将继续成为不对称催化和合成领域中的热门话题。在本报告中总结的我们的努力,不仅将为今后发展基于吲哚基手性杂环的有机催化不对称合成的创新策略打开一扇窗,而且还将激发全世界的化学家应对这一领域中的剩余挑战并推动进一步的发展。


























 京公网安备 11010802027423号
京公网安备 11010802027423号