当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Order, Disorder, and Reorder State of Lysozyme: Aggregation Mechanism by Raman Spectroscopy.
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpcb.9b09139 Sandip Dolui 1 , Animesh Mondal 1 , Anupam Roy 1 , Uttam Pal 1 , Supriya Das 1 , Achintya Saha 2 , Nakul C Maiti 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2019-12-27 , DOI: 10.1021/acs.jpcb.9b09139 Sandip Dolui 1 , Animesh Mondal 1 , Anupam Roy 1 , Uttam Pal 1 , Supriya Das 1 , Achintya Saha 2 , Nakul C Maiti 1
Affiliation
|
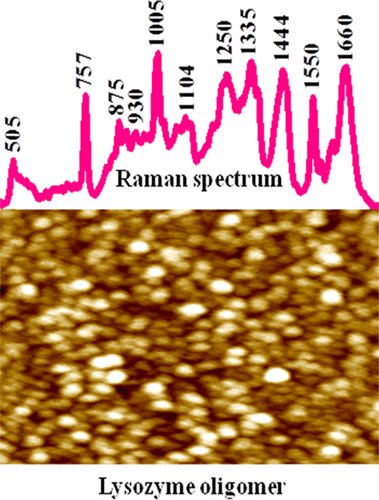
Lysozyme, like many other well-folded globular proteins, under stressful conditions produces nanoscale oligomer assembly and amyloid-like fibrillar aggregates. With engaging Raman microscopy, we made a critical structural analysis of oligomer and other assembly structures of lysozyme obtained from hen egg white and provided a quantitative estimation of a protein secondary structure in different states of its fibrillation. A strong amide I Raman band at 1660 cm-1 and a N-Cα-C stretching band at ∼930 cm-1 clearly indicated the presence of a substantial amount of α-helical folds of the protein in its oligomeric assembly state. In addition, analysis of the amide III region and Raman difference spectra suggested an ample presence of a PPII-like secondary structure in these oligomers without causing major loss of α-helical folds, which is found in the case of monomeric samples. Circular dichroism study also revealed the presence of typical α-helical folds in the oligomeric state. Nonetheless, most of the Raman bands associated with aromatic residues and disulfide (-S-S-) linkages broadened in the oligomeric state and indicated a collapse in the tertiary structure. In the fibrillar state of assembly, the amide I band became much sharper and enriched with the β-sheet secondary structure. Also, the disulfide bond vibration in matured fibrils became much weaker compared to monomer and oligomers and thus confirmed certain loss/cleavage of this bond during fibrillation. The Raman band of tryptophan and tyrosine residues indicated that some of these residues experienced a greater hydrophobic microenvironment in the fibrillar state than the protein in the oligomeric state of the assembly structure.
中文翻译:

溶菌酶的有序,无序和重排状态:拉曼光谱的聚集机制。
像许多其他折叠得很好的球状蛋白一样,溶菌酶在压力条件下也会产生纳米级寡聚物组装体和淀粉样纤维状聚集体。借助拉曼显微镜,我们对从蛋清获得的溶菌酶的低聚物和其他组装结构进行了关键的结构分析,并定量评估了其原纤维形成不同状态下的蛋白质二级结构。在1660 cm-1处有很强的酰胺I拉曼谱带,在约930 cm-1处有N-Cα-C拉伸谱带,清楚表明该蛋白在其寡聚组装状态下存在大量的α-螺旋折叠。此外,对酰胺III区和拉曼光谱的分析表明,这些低聚物中大量存在PPII样二级结构,而不会造成α-螺旋折叠的重大损失,这是在单体样品中发现的。圆二色性研究还揭示了低聚状态下典型的α-螺旋折叠的存在。但是,大多数与芳香族残基和二硫键(-SS-)相关的拉曼谱带在低聚状态下变宽,并表明三级结构塌陷。在纤维状的组装状态下,酰胺I谱带变得更加清晰,并富含β-折叠二级结构。而且,与单体和低聚物相比,成熟的原纤维中的二硫键振动变得更弱,因此证实了在原纤化过程中该键的某些损失/断裂。
更新日期:2019-12-27
中文翻译:

溶菌酶的有序,无序和重排状态:拉曼光谱的聚集机制。
像许多其他折叠得很好的球状蛋白一样,溶菌酶在压力条件下也会产生纳米级寡聚物组装体和淀粉样纤维状聚集体。借助拉曼显微镜,我们对从蛋清获得的溶菌酶的低聚物和其他组装结构进行了关键的结构分析,并定量评估了其原纤维形成不同状态下的蛋白质二级结构。在1660 cm-1处有很强的酰胺I拉曼谱带,在约930 cm-1处有N-Cα-C拉伸谱带,清楚表明该蛋白在其寡聚组装状态下存在大量的α-螺旋折叠。此外,对酰胺III区和拉曼光谱的分析表明,这些低聚物中大量存在PPII样二级结构,而不会造成α-螺旋折叠的重大损失,这是在单体样品中发现的。圆二色性研究还揭示了低聚状态下典型的α-螺旋折叠的存在。但是,大多数与芳香族残基和二硫键(-SS-)相关的拉曼谱带在低聚状态下变宽,并表明三级结构塌陷。在纤维状的组装状态下,酰胺I谱带变得更加清晰,并富含β-折叠二级结构。而且,与单体和低聚物相比,成熟的原纤维中的二硫键振动变得更弱,因此证实了在原纤化过程中该键的某些损失/断裂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号