当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Regioselective β-Arylation of α-Angelica Lactone through Isomerization/Addition under Mild Conditions.
ChemSusChem ( IF 8.4 ) Pub Date : 2019-12-10 , DOI: 10.1002/cssc.201902761 Kai-Feng Zhuo 1 , Shang-Hai Yu 1 , Tian-Jun Gong 1 , Yao Fu 1
ChemSusChem ( IF 8.4 ) Pub Date : 2019-12-10 , DOI: 10.1002/cssc.201902761 Kai-Feng Zhuo 1 , Shang-Hai Yu 1 , Tian-Jun Gong 1 , Yao Fu 1
Affiliation
|
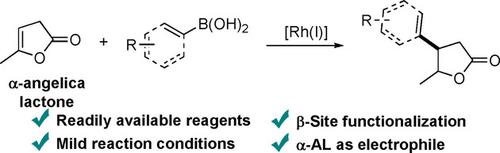
The conversion of biomass-based platform molecules into various high-value chemicals greatly promotes the utilization of renewable biomass resources. Herein, an example of Rh-catalyzed β-arylation of levulinic-acid-derived α-angelica lactone was reported, providing the γ-lactone-structure products with high regioselectivity. Both arylboronic and alkenylboronic acids could be applied in this transformation. This reaction tolerated a variety of synthetically important functional groups. Moreover, the obtained γ-lactone products could be readily converted to high-value products such as 1,4-diols and γ-methoxy-carboxylates.
中文翻译:

在温和条件下通过异构化/加成反应使α-当归内酯的区域选择性β-芳基化。
基于生物质的平台分子向各种高价值化学品的转化极大地促进了可再生生物质资源的利用。在此,报道了由乙酰丙酸衍生的α-当归内酯的Rh催化的β-芳基化的例子,其提供了具有高区域选择性的γ-内酯结构的产物。芳基硼酸和烯基硼酸均可用于该转化中。该反应耐受各种合成上重要的官能团。而且,所获得的γ-内酯产物可以容易地转化为高价值产物,例如1,4-二醇和γ-甲氧基-羧酸盐。
更新日期:2020-01-23
中文翻译:

在温和条件下通过异构化/加成反应使α-当归内酯的区域选择性β-芳基化。
基于生物质的平台分子向各种高价值化学品的转化极大地促进了可再生生物质资源的利用。在此,报道了由乙酰丙酸衍生的α-当归内酯的Rh催化的β-芳基化的例子,其提供了具有高区域选择性的γ-内酯结构的产物。芳基硼酸和烯基硼酸均可用于该转化中。该反应耐受各种合成上重要的官能团。而且,所获得的γ-内酯产物可以容易地转化为高价值产物,例如1,4-二醇和γ-甲氧基-羧酸盐。


























 京公网安备 11010802027423号
京公网安备 11010802027423号