当前位置:
X-MOL 学术
›
Chem. Asian J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Facile Synthesis of Polysubstituted Indolizines via One-Pot Reaction of 1-Acetylaryl 2-Formylpyrroles and Enals.
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2019-12-27 , DOI: 10.1002/asia.201901517 Minghua Gong 1 , Jingcheng Guo 1 , Pengrui Jiang 1 , Ye Zhang 1 , Zhenqian Fu 1, 2 , Wei Huang 1, 2
Chemistry - An Asian Journal ( IF 4.1 ) Pub Date : 2019-12-27 , DOI: 10.1002/asia.201901517 Minghua Gong 1 , Jingcheng Guo 1 , Pengrui Jiang 1 , Ye Zhang 1 , Zhenqian Fu 1, 2 , Wei Huang 1, 2
Affiliation
|
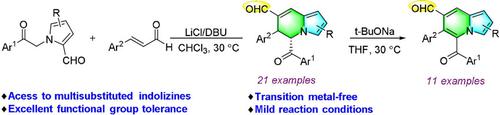
An efficient method for the synthesis of polysubstituted indolizines has been developed based on formal [4+2] annulation of 1-acetylaryl 2-formylpyrroles with enals, followed by oxidative aromatization. Pyridine-type six-membered rings were constructed in this transformation. This transition metal-free reaction features mild reaction conditions, a broad substrate scope, and excellent functional group tolerance. Notably, the formyl group is well tolerated under reaction conditions.
中文翻译:

通过1-乙酰基芳基2-甲酰基吡咯和Enals的一锅反应轻松合成多取代的吲哚嗪。
基于1-乙酰芳基2-甲酰基吡咯与烯醛的正式[4 + 2]环合,然后进行氧化芳构化,已经开发了一种有效的合成多取代的吲哚嗪的方法。在该转化中构建了吡啶型六元环。这种无过渡金属的反应具有温和的反应条件,广泛的底物范围和出色的官能团耐受性。值得注意的是,甲酰基在反应条件下具有良好的耐受性。
更新日期:2019-12-27
中文翻译:

通过1-乙酰基芳基2-甲酰基吡咯和Enals的一锅反应轻松合成多取代的吲哚嗪。
基于1-乙酰芳基2-甲酰基吡咯与烯醛的正式[4 + 2]环合,然后进行氧化芳构化,已经开发了一种有效的合成多取代的吲哚嗪的方法。在该转化中构建了吡啶型六元环。这种无过渡金属的反应具有温和的反应条件,广泛的底物范围和出色的官能团耐受性。值得注意的是,甲酰基在反应条件下具有良好的耐受性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号