当前位置:
X-MOL 学术
›
Biosens. Bioelectron.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exosomes-mediated synthetic Dicer substrates delivery for intracellular Dicer imaging detection.
Biosensors and Bioelectronics ( IF 12.6 ) Pub Date : 2019-12-09 , DOI: 10.1016/j.bios.2019.111907 Wenhao Dai 1 , Lei Su 1 , Huiting Lu 2 , Haifeng Dong 1 , Xueji Zhang 3
Biosensors and Bioelectronics ( IF 12.6 ) Pub Date : 2019-12-09 , DOI: 10.1016/j.bios.2019.111907 Wenhao Dai 1 , Lei Su 1 , Huiting Lu 2 , Haifeng Dong 1 , Xueji Zhang 3
Affiliation
|
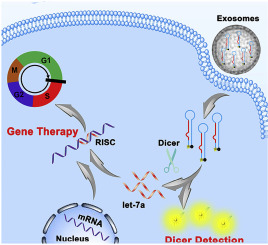
Ribonuclease Dicer initiates gene-silencing process by cleaving exogenously long RNA duplexes into small interfering RNA (siRNA) or endogenous precursor microRNAs (pre-miRNAs) into mature miRNAs. It holds great promise in cancer diagnosis and therapeutics due to its molecular ruler role. However, the intracellular Dicer detection remains a key challenge and Dicer related gene therapy has never been explored. In this study, we design a fluorescent labeling Dicer substrate and effectively deliver it into cell by exosomes derived from the target parent cells for intracellular Dicer expression level monitor and gene therapy. Using pre-miRNA let-7a as a model, the Dicer substrates with two terminals labeled with fluorescent and quencher group respectively was obtained by T4 RNA mediated ligase reaction from two short RNA sequences. Then, the substrate was packaged into exosomes by electroporation and delivered to target cells for intracellular dicer imaging detection. After packaging substrates into exosomes with little immunogenicity and good innate biocompatibility by electroporation and delivered to target cells, the Dicer mediated substrate cleavage was effectively monitored by the fluorescence recovery, providing a powerful tool for Dicer analysis. Importantly, the cleaved product exhibited significant suppression toward tumor cell growth and regulated cancer cells cycle. This work might open a new avenue for Dicer analysis and Dicer-related clinical application.
中文翻译:

外来体介导的合成Dicer底物递送,用于细胞内Dicer成像检测。
核糖核酸酶切酶通过将外源性长RNA双链体切割成小的干扰RNA(siRNA)或内源性前体microRNA(pre-miRNA)裂解成成熟的miRNA来启动基因沉默过程。由于其分子尺的作用,它在癌症诊断和治疗中具有广阔的前景。然而,细胞内Dicer的检测仍然是关键的挑战,并且从未探索与Dicer相关的基因疗法。在这项研究中,我们设计了一种荧光标记的Dicer底物,并通过来自目标亲本细胞的外泌体有效地将其递送到细胞中,以进行细胞内Dicer表达水平监测和基因治疗。以pre-miRNA let-7a为模型,通过T4 RNA介导的连接酶反应,从两个短RNA序列中分别获得了两个末端分别标记有荧光基团和淬灭基团的Dicer底物。然后,通过电穿孔将底物包装到外泌体中,并输送至靶细胞进行细胞内切丁酶成像检测。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。
更新日期:2019-12-11
中文翻译:

外来体介导的合成Dicer底物递送,用于细胞内Dicer成像检测。
核糖核酸酶切酶通过将外源性长RNA双链体切割成小的干扰RNA(siRNA)或内源性前体microRNA(pre-miRNA)裂解成成熟的miRNA来启动基因沉默过程。由于其分子尺的作用,它在癌症诊断和治疗中具有广阔的前景。然而,细胞内Dicer的检测仍然是关键的挑战,并且从未探索与Dicer相关的基因疗法。在这项研究中,我们设计了一种荧光标记的Dicer底物,并通过来自目标亲本细胞的外泌体有效地将其递送到细胞中,以进行细胞内Dicer表达水平监测和基因治疗。以pre-miRNA let-7a为模型,通过T4 RNA介导的连接酶反应,从两个短RNA序列中分别获得了两个末端分别标记有荧光基团和淬灭基团的Dicer底物。然后,通过电穿孔将底物包装到外泌体中,并输送至靶细胞进行细胞内切丁酶成像检测。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。通过电穿孔将底物包装到具有很少免疫原性和良好的先天生物相容性的外泌体中并递送到靶细胞后,Dicer介导的底物裂解可通过荧光恢复得到有效监测,为Dicer分析提供了强大的工具。重要的是,裂解产物显示出对肿瘤细胞生长的显着抑制和调节的癌细胞周期。这项工作可能会为Dicer分析和与Dicer相关的临床应用开辟新的途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号