Applied Catalysis A: General ( IF 5.5 ) Pub Date : 2019-12-06 , DOI: 10.1016/j.apcata.2019.117372 Tianyu Cao , Renjing Huang , Raymond J. Gorte , John M. Vohs
|
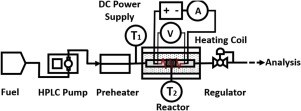
Cooling of critical engine components is important for hypersonic aircraft and endothermic reforming reactions on the fuel could play an important role by taking up some of the heat. Here, we propose using alkylamines that dehydrogenate to nitriles and H2 over ZrO2 catalysts as the endothermic fuel. Temperature programmed desorption (TPD) revealed that 1-propanamine on ZrO2 undergoes dehydrogenation at temperatures below 600 K. At 723 K and below, the selectivity to form H2 and propionitrile is greater than 90%. Heat measurements at 60 bar show heat uptakes as large as 2000 kJ kg-1 of fuel reacted could be achieved at 723 K. Side reactions at higher conversions and temperatures lowered this value but values above 1000 kJ kg-1 were still obtained at nearly 100% conversion of the amine. The observation of a significant endothermic effect suggests that the unconventional functional groups in the fuels merit further investigation to achieve a more profound chemical heat sink. Possible ways to apply this approach are discussed.
中文翻译:

1-丙胺在氧化锆催化剂上的吸热反应
关键发动机部件的冷却对于高超音速飞机很重要,并且通过吸收一些热量,燃料上的吸热重整反应可能会发挥重要作用。在这里,我们建议使用在ZrO 2催化剂上脱氢为腈和H 2的烷基胺作为吸热燃料。程序升温脱附(TPD)表明ZrO 2上的1-丙胺在低于600 K的温度下进行脱氢。在723 K及以下的温度下,形成H 2和丙腈的选择性大于90%。在60 bar的热量测量结果显示,热量吸收高达2000 kJ kg -1在723 K的温度下可以达到100%的反应燃料。在较高的转化率和温度下,副反应降低了该值,但在胺的转化率接近100%时仍获得了1000 kJ kg -1以上的值。观察到明显的吸热效应表明,燃料中非常规官能团值得进一步研究以实现更深的化学散热。讨论了应用此方法的可能方法。



























 京公网安备 11010802027423号
京公网安备 11010802027423号