当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Aromaticity and tautomerism of a 4n π electron dihydrohexaazapentacene
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/06 , DOI: 10.1039/c9qo01285k Chun-Lin Sun 1, 2, 3, 4, 5 , Xiao-E Luo 1, 2, 3, 4, 5 , Huan Xu 1, 2, 3, 4, 5 , Qi-Wei Song 1, 2, 3, 4, 5 , Zhi-Ping Fan 1, 2, 3, 4, 5 , Xiao-Zhen Wang 1, 2, 3, 4, 5 , Jing-Jing Cao 1, 2, 3, 4, 5 , Zi-Fa Shi 1, 2, 3, 4, 5 , Hao-Li Zhang 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2019/12/06 , DOI: 10.1039/c9qo01285k Chun-Lin Sun 1, 2, 3, 4, 5 , Xiao-E Luo 1, 2, 3, 4, 5 , Huan Xu 1, 2, 3, 4, 5 , Qi-Wei Song 1, 2, 3, 4, 5 , Zhi-Ping Fan 1, 2, 3, 4, 5 , Xiao-Zhen Wang 1, 2, 3, 4, 5 , Jing-Jing Cao 1, 2, 3, 4, 5 , Zi-Fa Shi 1, 2, 3, 4, 5 , Hao-Li Zhang 1, 2, 3, 4, 5
Affiliation
|
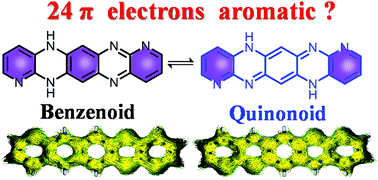
Tautomerism is an interesting phenomenon that allows different properties to coexist in the same molecule. However, the understanding of tautomerism of large antiaromatic skeletons is very limited. We report herein the intriguing tautomerism behaviors of a new hexazapentacene derivative, named DHHAP. In solution, DHHAP exists as a mixture of benzenoid and quinonoid tautomers in a rough ratio of 1 : 1. DFT calulations reveal that DHHAP is slightly more stable than its 4n + 2π hexazapentacene counterpart although it has 4n π electrons. DHHAP exhibits different halochromic behaviors upon addition of strong and mild acids. Despite multiple protonation possibilities, careful NMR investigation revealed that only one di-protonation process occurs at symmetrical sites on the quinonoid structure, which is attributed to electrostatic potential-induced protonation regioselectivity. Femtosecond transient absorption (fs-TA) spectroscopy revealed different excited state dynamics of DHHAP-related species. Deep understanding of the tautomerism and aromaticity of dihydrohexazapentacene may pave a new way for the design of stable 4n π heteroacenes towards novel organic semiconductors and sensing materials.
中文翻译:

4nπ电子二氢六氮杂并五苯的芳香性和互变异构
互变异构现象是一种有趣的现象,它使不同的特性共存于同一分子中。但是,对大的抗芳香族骨架的互变异构现象的理解非常有限。我们在这里报告了一种新的名为DHHAP的六氮杂并五苯衍生物的有趣的互变异构行为。在溶液中,DHHAP存在如苯和醌型互变异构体以1:1的粗比的混合物:1. DFT calulations揭示DHHAP稍微更稳定比其第4n +2πhexazapentacene对方尽管它具有4Nπ电子。脱氢表雄酮加入强酸和温和酸后,会表现出不同的盐致变色行为。尽管存在多种质子化可能性,但仔细的NMR研究表明,在醌型结构的对称位点上仅发生一种双质子化过程,这归因于静电势诱导的质子化区域选择性。飞秒瞬态吸收(fs-TA)光谱揭示了DHHAP相关物种的不同激发态动力学。对二氢六氮杂戊并苯的互变异构性和芳香性的深入了解可能为稳定的4nπ杂并苯向新型有机半导体和传感材料的设计铺平了新途径。
更新日期:2020-02-13
中文翻译:

4nπ电子二氢六氮杂并五苯的芳香性和互变异构
互变异构现象是一种有趣的现象,它使不同的特性共存于同一分子中。但是,对大的抗芳香族骨架的互变异构现象的理解非常有限。我们在这里报告了一种新的名为DHHAP的六氮杂并五苯衍生物的有趣的互变异构行为。在溶液中,DHHAP存在如苯和醌型互变异构体以1:1的粗比的混合物:1. DFT calulations揭示DHHAP稍微更稳定比其第4n +2πhexazapentacene对方尽管它具有4Nπ电子。脱氢表雄酮加入强酸和温和酸后,会表现出不同的盐致变色行为。尽管存在多种质子化可能性,但仔细的NMR研究表明,在醌型结构的对称位点上仅发生一种双质子化过程,这归因于静电势诱导的质子化区域选择性。飞秒瞬态吸收(fs-TA)光谱揭示了DHHAP相关物种的不同激发态动力学。对二氢六氮杂戊并苯的互变异构性和芳香性的深入了解可能为稳定的4nπ杂并苯向新型有机半导体和传感材料的设计铺平了新途径。


























 京公网安备 11010802027423号
京公网安备 11010802027423号