当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photo-induced thiol-ene reactions for late-stage functionalization of unsaturated polyether macrocycles: regio and diastereoselective access to macrocyclic dithiol derivatives.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02375e Elodie Brun 1 , Ke-Feng Zhang , Laure Guénée , Jérôme Lacour
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02375e Elodie Brun 1 , Ke-Feng Zhang , Laure Guénée , Jérôme Lacour
Affiliation
|
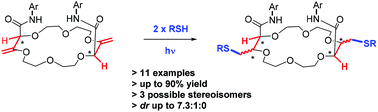
Double hydrothiolation of bis enol ether macrocycles was achieved under photo-mediated conditions. The thiol-ene reactions afford a fully regioselective anti-Markovnikov post-functionalization. Thanks to the use of ethanedithiol as reagent, moderate to excellent diastereoselectivity was accomplished leading to macrocycles containing four defined stereocenters in only three steps from 1,4-dioxane, tetrahydrofuran (THF) or tetrahydropyran (THP).
中文翻译:

用于不饱和聚醚大环化合物后期官能化的光诱导硫醇-烯反应:区域和非对映选择性进入大环二硫醇衍生物。
双烯醇醚大环的双氢硫醇化在光介导的条件下实现。硫醇-烯反应提供了完全区域选择性的反马尔科夫尼科夫后功能化。由于使用了乙二硫醇作为试剂,因此实现了中度至出色的非对映选择性,从而使大环在三个步骤中仅由1,4-二恶烷,四氢呋喃(THF)或四氢吡喃(THP)包含四个定义的立体中心。
更新日期:2020-01-15
中文翻译:

用于不饱和聚醚大环化合物后期官能化的光诱导硫醇-烯反应:区域和非对映选择性进入大环二硫醇衍生物。
双烯醇醚大环的双氢硫醇化在光介导的条件下实现。硫醇-烯反应提供了完全区域选择性的反马尔科夫尼科夫后功能化。由于使用了乙二硫醇作为试剂,因此实现了中度至出色的非对映选择性,从而使大环在三个步骤中仅由1,4-二恶烷,四氢呋喃(THF)或四氢吡喃(THP)包含四个定义的立体中心。



























 京公网安备 11010802027423号
京公网安备 11010802027423号