当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Betaine–N‐Heterocyclic Carbene Interconversions of Quinazolin‐4‐One Imidazolium Mesomeric Betaines. Sulfur, Selenium, and Borane Adduct Formation
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-20 , DOI: 10.1002/ejoc.201901622 Sergey Deev 1 , Sviatoslav Batsyts 2 , Ekaterina Sheina 1 , Tatyana S. Shestakova 1 , Igor Khalimbadzha 1 , Mikhail A. Kiskin 3 , Valery Charushin 1, 4 , Oleg Chupakhin 1, 4 , Alexander S. Paramonov 5 , Zakhar O. Shenkarev 5 , Jan C. Namyslo 2 , Andreas Schmidt 2
European Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2020-01-20 , DOI: 10.1002/ejoc.201901622 Sergey Deev 1 , Sviatoslav Batsyts 2 , Ekaterina Sheina 1 , Tatyana S. Shestakova 1 , Igor Khalimbadzha 1 , Mikhail A. Kiskin 3 , Valery Charushin 1, 4 , Oleg Chupakhin 1, 4 , Alexander S. Paramonov 5 , Zakhar O. Shenkarev 5 , Jan C. Namyslo 2 , Andreas Schmidt 2
Affiliation
|
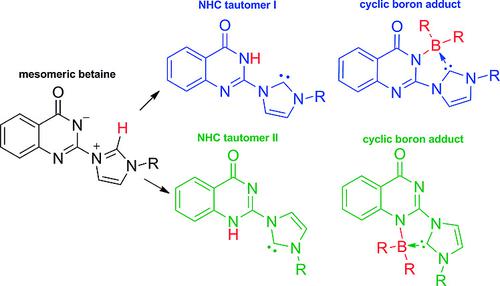
The tautomers of quinazolinonide‐substituted imidazolium betaines are N‐heterocyclic carbenes which form cyclic boron adducts as well as thiones and selenones. The anionic substituent allowed for the examination of electronic effects on the carbene carbon atom, among others, by 77Se NMR measurements.

中文翻译:

喹唑啉-4-一咪唑甜美甜菜碱的甜菜碱-N-杂环碳素相互转化。硫,硒和硼烷加合物的形成
喹唑啉酮取代的咪唑鎓甜菜碱的互变异构体是N-杂环卡宾,它们形成环状硼加合物以及硫酮和硒酮。阴离子取代基允许通过77 Se NMR测量来检查对卡宾碳原子的电子效应。

更新日期:2020-01-21

中文翻译:

喹唑啉-4-一咪唑甜美甜菜碱的甜菜碱-N-杂环碳素相互转化。硫,硒和硼烷加合物的形成
喹唑啉酮取代的咪唑鎓甜菜碱的互变异构体是N-杂环卡宾,它们形成环状硼加合物以及硫酮和硒酮。阴离子取代基允许通过77 Se NMR测量来检查对卡宾碳原子的电子效应。




























 京公网安备 11010802027423号
京公网安备 11010802027423号