当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
N-Terminal Region of Vibrio parahemolyticus Thermostable Direct Hemolysin Regulates the Membrane-Damaging Action of the Toxin.
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.biochem.9b00937 Nidhi Kundu 1 , Pratima Verma 1 , Anil Kumar 2 , Vinica Dhar 3 , Somnath Dutta 2 , Kausik Chattopadhyay 1
Biochemistry ( IF 2.9 ) Pub Date : 2019-12-20 , DOI: 10.1021/acs.biochem.9b00937 Nidhi Kundu 1 , Pratima Verma 1 , Anil Kumar 2 , Vinica Dhar 3 , Somnath Dutta 2 , Kausik Chattopadhyay 1
Affiliation
|
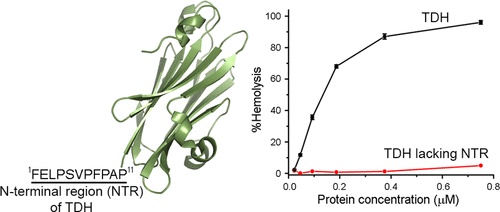
Thermostable direct hemolysin (TDH) of Vibrio parahemolyticus is a membrane-damaging pore-forming toxin with potent cytolytic/cytotoxic activity. TDH exists as a tetramer consisting of protomers with a core β-sandwich domain, flanked by an 11-amino acid long N-terminal region (NTR). This NTR could not be modeled in the previously determined crystal structure of TDH. Moreover, the functional implication of NTR for the membrane-damaging action of TDH remains unknown. In the present study, we have explored the implications of NTR for the structure-function mechanism of TDH. Our data show that the presence of NTR modulates the physicochemical property of TDH in terms of augmenting the amyloidogenic propensity of the protein. Deletion of NTR compromises the binding of TDH toward target cell membranes and drastically affects the membrane-damaging cytolytic/cytotoxic activity of the toxin. Mutations of aromatic/hydrophobic residues within NTR also confer compromised cell-killing activity. Moreover, covalent trapping of NTR, via an engineered disulfide bond, against the core β-sandwich domain also abrogates the cytolytic/cytotoxic activity of TDH. This observation suggests that an unrestrained configuration of NTR is crucial for the membrane-damaging action of TDH. On the basis of our study, we propose a model explaining the role of NTR in the membrane-damaging function of TDH.
中文翻译:

副溶血性弧菌的N端区域热稳定的直接溶血素调节毒素的膜破坏作用。
副溶血性弧菌的热稳定直接溶血素(TDH)是一种破坏膜的成孔毒素,具有有效的细胞溶解/细胞毒活性。TDH以由具有核心β-三明治结构域的启动子组成的四聚体形式存在,其侧翼是11个氨基酸长的N端区域(NTR)。无法在先前确定的TDH晶体结构中模拟该NTR。而且,NTR对TDH的膜破坏作用的功能含义仍是未知的。在本研究中,我们探索了NTR对TDH的结构功能机制的影响。我们的数据表明,NTR的存在可调节TDH的理化特性,从而增强该蛋白的淀粉样变性倾向。NTR的缺失损害了TDH与靶细胞膜的结合,并极大地影响了毒素的膜破坏性细胞溶解/细胞毒性活性。NTR中芳族/疏水残基的突变也赋予受损的细胞杀伤活性。此外,通过工程化的二硫键对核心β-三明治结构域的NTR共价捕集也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。通过改造的二硫键,针对核心β-三明治结构域也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。通过改造的二硫键,针对核心β-三明治结构域也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。
更新日期:2019-12-20
中文翻译:

副溶血性弧菌的N端区域热稳定的直接溶血素调节毒素的膜破坏作用。
副溶血性弧菌的热稳定直接溶血素(TDH)是一种破坏膜的成孔毒素,具有有效的细胞溶解/细胞毒活性。TDH以由具有核心β-三明治结构域的启动子组成的四聚体形式存在,其侧翼是11个氨基酸长的N端区域(NTR)。无法在先前确定的TDH晶体结构中模拟该NTR。而且,NTR对TDH的膜破坏作用的功能含义仍是未知的。在本研究中,我们探索了NTR对TDH的结构功能机制的影响。我们的数据表明,NTR的存在可调节TDH的理化特性,从而增强该蛋白的淀粉样变性倾向。NTR的缺失损害了TDH与靶细胞膜的结合,并极大地影响了毒素的膜破坏性细胞溶解/细胞毒性活性。NTR中芳族/疏水残基的突变也赋予受损的细胞杀伤活性。此外,通过工程化的二硫键对核心β-三明治结构域的NTR共价捕集也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。通过改造的二硫键,针对核心β-三明治结构域也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。通过改造的二硫键,针对核心β-三明治结构域也消除了TDH的细胞溶解/细胞毒性活性。该观察结果表明,不受约束的NTR构型对于TDH的膜破坏作用至关重要。在我们的研究基础上,我们提出了一个模型,解释了NTR在TDH的膜破坏功能中的作用。



























 京公网安备 11010802027423号
京公网安备 11010802027423号