当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Crucial Roles of Two Hydrated Mg2+ Ions in Reaction Catalysis of the Pistol Ribozyme.
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/anie.201912522 Marianna Teplova 1 , Christoph Falschlunger 2 , Olga Krasheninina 2 , Michaela Egger 2 , Aiming Ren 3 , Dinshaw J Patel 1 , Ronald Micura 2
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2020-01-09 , DOI: 10.1002/anie.201912522 Marianna Teplova 1 , Christoph Falschlunger 2 , Olga Krasheninina 2 , Michaela Egger 2 , Aiming Ren 3 , Dinshaw J Patel 1 , Ronald Micura 2
Affiliation
|
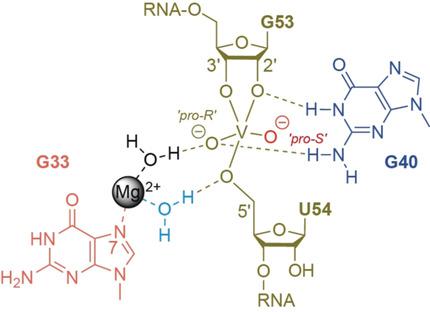
Pistol ribozymes constitute a new class of small self‐cleaving RNAs. Crystal structures have been solved, providing three‐dimensional snapshots along the reaction coordinate of pistol phosphodiester cleavage, corresponding to the pre‐catalytic state, a vanadate mimic of the transition state, and the product. The results led to the proposed underlying chemical mechanism. Importantly, a hydrated Mg2+ ion remains innersphere‐coordinated to N7 of G33 in all three states, and is consistent with its likely role as acid in general acid base catalysis (δ and β catalysis). Strikingly, the new structures shed light on a second hydrated Mg2+ ion that approaches the scissile phosphate from its binding site in the pre‐cleavage state to reach out for water‐mediated hydrogen bonding in the cyclophosphate product. The major role of the second Mg2+ ion appears to be the stabilization of product conformation. This study delivers a mechanistic understanding of ribozyme‐catalyzed backbone cleavage.
中文翻译:

两种水合Mg2 +离子在手枪核酶反应催化中的关键作用。
手枪核酶构成了一类新的小分子自切割RNA。晶体结构已解决,沿着手枪磷酸二酯裂解的反应坐标提供了三维快照,对应于催化前状态,过渡态的钒酸盐模拟物和产物。结果导致提出了潜在的化学机理。重要的是,在所有三种状态下,一个水合的Mg 2+离子在内部球体内均与G33的N7配位,并且与它在一般酸碱催化(δ和β催化)中作为酸的可能作用相一致。引人注目的是,新结构在第二种水合Mg 2+上产生了亮光。离子在裂解前从其结合位点接近可裂解的磷酸根,到达环磷酸酯产物中水介导的氢键。第二个Mg 2+离子的主要作用似乎是产物构象的稳定。这项研究提供了对核酶催化的主链裂解的机械理解。
更新日期:2020-01-10
中文翻译:

两种水合Mg2 +离子在手枪核酶反应催化中的关键作用。
手枪核酶构成了一类新的小分子自切割RNA。晶体结构已解决,沿着手枪磷酸二酯裂解的反应坐标提供了三维快照,对应于催化前状态,过渡态的钒酸盐模拟物和产物。结果导致提出了潜在的化学机理。重要的是,在所有三种状态下,一个水合的Mg 2+离子在内部球体内均与G33的N7配位,并且与它在一般酸碱催化(δ和β催化)中作为酸的可能作用相一致。引人注目的是,新结构在第二种水合Mg 2+上产生了亮光。离子在裂解前从其结合位点接近可裂解的磷酸根,到达环磷酸酯产物中水介导的氢键。第二个Mg 2+离子的主要作用似乎是产物构象的稳定。这项研究提供了对核酶催化的主链裂解的机械理解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号