当前位置:
X-MOL 学术
›
J. Inorg. Biochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structural zinc binding sites shaped for greater works: Structure-function relations in classical zinc finger, hook and clasp domains.
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.jinorgbio.2019.110955 Michał Padjasek 1 , Anna Kocyła 1 , Katarzyna Kluska 1 , Olga Kerber 1 , Józef Ba Tran 1 , Artur Krężel 1
Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2019-12-05 , DOI: 10.1016/j.jinorgbio.2019.110955 Michał Padjasek 1 , Anna Kocyła 1 , Katarzyna Kluska 1 , Olga Kerber 1 , Józef Ba Tran 1 , Artur Krężel 1
Affiliation
|
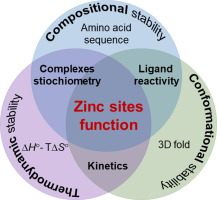
Metal ions are essential elements present in biological systems able to facilitate many cellular processes including proliferation, signaling, DNA synthesis and repair. Zinc ion (Zn(II)) is an important cofactor for numerous biochemical reactions. Commonly, structural zinc sites demonstrate high Zn(II) affinity and compact architecture required for sequence-specific macromolecule binding. However, how Zn(II)-dependent proteins fold, how their dissociation occurs, and which factors modulate zinc protein affinity as well as stability remains not fully understood. The molecular rules governing precise regulation of zinc proteins function are hidden in the relationship between sequence and structure, and hence require deep understanding of their folding mechanism under metal load, reactivity and metal-to-protein affinity. Even though, this sequence-structure relationship has an impact on zinc proteins function, it has been shown that other biological factors including cellular localization and Zn(II) availability influence overall protein behavior. Taking into account all of the mentioned factors, in this review, we aim to describe the relationship between structure-function-stability of zinc structural sites, found in a zinc finger, zinc hook and zinc clasps, and reach far beyond a structural point of view in order to appreciate the balance between chemistry and biology that govern the protein world.
中文翻译:

结构锌结合位点的形状适合更大的工作:经典锌指,钩和扣结构域中的结构-功能关系。
金属离子是生物系统中必需的元素,能够促进许多细胞过程,包括增殖,信号传导,DNA合成和修复。锌离子(Zn(II))是许多生化反应的重要辅助因子。通常,结构锌位点表现出高的Zn(II)亲和力和序列特异性大分子结合所需的紧凑结构。但是,Zn(II)依赖性蛋白如何折叠,如何解离以及调节锌蛋白亲和性和稳定性的因素仍未完全了解。调控锌蛋白功能的分子规则隐藏在序列与结构之间的关系中,因此需要深入了解其在金属负载,反应性和金属对蛋白亲和力下的折叠机理。虽然,这种序列-结构关系对锌蛋白的功能有影响,已显示包括细胞定位和Zn(II)可用性在内的其他生物学因素会影响蛋白的整体行为。考虑到所有上述因素,在本综述中,我们旨在描述锌指,锌钩和锌钩中发现的锌结构位点的结构-功能-稳定性之间的关系,并且其作用范围远远超出了以了解控制蛋白质世界的化学和生物学之间的平衡。
更新日期:2019-12-05
中文翻译:

结构锌结合位点的形状适合更大的工作:经典锌指,钩和扣结构域中的结构-功能关系。
金属离子是生物系统中必需的元素,能够促进许多细胞过程,包括增殖,信号传导,DNA合成和修复。锌离子(Zn(II))是许多生化反应的重要辅助因子。通常,结构锌位点表现出高的Zn(II)亲和力和序列特异性大分子结合所需的紧凑结构。但是,Zn(II)依赖性蛋白如何折叠,如何解离以及调节锌蛋白亲和性和稳定性的因素仍未完全了解。调控锌蛋白功能的分子规则隐藏在序列与结构之间的关系中,因此需要深入了解其在金属负载,反应性和金属对蛋白亲和力下的折叠机理。虽然,这种序列-结构关系对锌蛋白的功能有影响,已显示包括细胞定位和Zn(II)可用性在内的其他生物学因素会影响蛋白的整体行为。考虑到所有上述因素,在本综述中,我们旨在描述锌指,锌钩和锌钩中发现的锌结构位点的结构-功能-稳定性之间的关系,并且其作用范围远远超出了以了解控制蛋白质世界的化学和生物学之间的平衡。



























 京公网安备 11010802027423号
京公网安备 11010802027423号