当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Role of the N-Terminal Transmembrane Helix Contacts in the Activation of FGFR3
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-12-05 , DOI: 10.1002/jcc.26122 Daisuke Matsuoka 1 , Motoshi Kamiya 2 , Takeshi Sato 3 , Yuji Sugita 1, 2, 4
Journal of Computational Chemistry ( IF 3 ) Pub Date : 2019-12-05 , DOI: 10.1002/jcc.26122 Daisuke Matsuoka 1 , Motoshi Kamiya 2 , Takeshi Sato 3 , Yuji Sugita 1, 2, 4
Affiliation
|
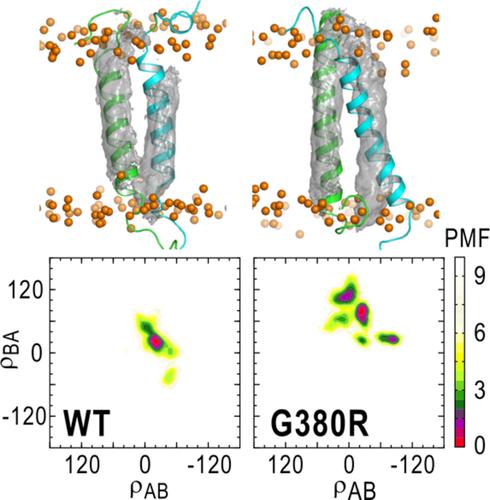
Fibroblast growth factor receptor 3 (FGFR3) is a member of receptor tyrosine kinases, which is involved in skeletal cell growth, differentiation, and migration. FGFR3 transduces biochemical signals from the extracellular ligand‐binding domain to the intracellular kinase domain through the conformational changes of the transmembrane (TM) helix dimer. Here, we apply generalized replica exchange with solute tempering method to wild type (WT) and G380R mutant (G380R) of FGFR3. The dimer interface in G380R is different from WT and the simulation results are in good agreement with the solid‐state nuclear magnetic resonance (NMR) spectroscopy. TM helices in G380R are extended more than WT, and thereby, G375 in G380R contacts near the N‐termini of the TM helix dimer. Considering that both G380R and G375C show the constitutive activation, the formation of the N‐terminal contacts of the TM helices can be generally important for the activation mechanism. © 2019 Wiley Periodicals, Inc.
中文翻译:

N 端跨膜螺旋接触在 FGFR3 激活中的作用
成纤维细胞生长因子受体 3 (FGFR3) 是受体酪氨酸激酶的成员,参与骨骼细胞的生长、分化和迁移。FGFR3 通过跨膜 (TM) 螺旋二聚体的构象变化将生化信号从细胞外配体结合域转导到细胞内激酶域。在这里,我们将具有溶质调温方法的广义复制品交换应用于 FGFR3 的野生型 (WT) 和 G380R 突变体 (G380R)。G380R 中的二聚体界面与 WT 不同,模拟结果与固态核磁共振 (NMR) 光谱非常吻合。G380R 中的 TM 螺旋比 WT 延伸得更多,因此,G380R 中的 G375 接触 TM 螺旋二聚体的 N 端附近。考虑到 G380R 和 G375C 都表现出本构激活,TM 螺旋 N 端接触的形成通常对激活机制很重要。© 2019 威利期刊公司。
更新日期:2019-12-05
中文翻译:

N 端跨膜螺旋接触在 FGFR3 激活中的作用
成纤维细胞生长因子受体 3 (FGFR3) 是受体酪氨酸激酶的成员,参与骨骼细胞的生长、分化和迁移。FGFR3 通过跨膜 (TM) 螺旋二聚体的构象变化将生化信号从细胞外配体结合域转导到细胞内激酶域。在这里,我们将具有溶质调温方法的广义复制品交换应用于 FGFR3 的野生型 (WT) 和 G380R 突变体 (G380R)。G380R 中的二聚体界面与 WT 不同,模拟结果与固态核磁共振 (NMR) 光谱非常吻合。G380R 中的 TM 螺旋比 WT 延伸得更多,因此,G380R 中的 G375 接触 TM 螺旋二聚体的 N 端附近。考虑到 G380R 和 G375C 都表现出本构激活,TM 螺旋 N 端接触的形成通常对激活机制很重要。© 2019 威利期刊公司。



























 京公网安备 11010802027423号
京公网安备 11010802027423号