当前位置:
X-MOL 学术
›
ChemBioChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Triazolecarbaldehyde Reagents for One-Step N-Terminal Protein Modification.
ChemBioChem ( IF 3.2 ) Pub Date : 2020-01-16 , DOI: 10.1002/cbic.201900692 Akira Onoda 1 , Nozomu Inoue 1 , Eigo Sumiyoshi 1 , Takashi Hayashi 1
ChemBioChem ( IF 3.2 ) Pub Date : 2020-01-16 , DOI: 10.1002/cbic.201900692 Akira Onoda 1 , Nozomu Inoue 1 , Eigo Sumiyoshi 1 , Takashi Hayashi 1
Affiliation
|
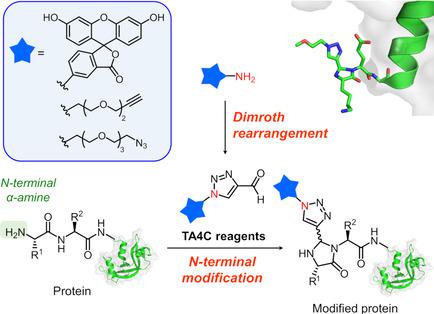
Site-specific modification of peptides and proteins is a key aspect of protein engineering. We developed a method for modification of the N terminus of proteins using 1H-1,2,3-triazole-4-carbaldehyde (TA4C) derivatives, which can be prepared in one step. The N-terminal specific labeling of bioactive peptides and proteins with the TA4C derivatives proceeds under mild reaction conditions in excellent conversion (angiotensin I: 92 %, ribonuclease A: 90 %). This method enables site-specific conjugation of various functional molecules such as fluorophores, biotin, and polyethylene glycol attached to the triazole ring to the N terminus. Furthermore, a functional molecule modified with a primary amine moiety can be directly converted into a TA4C derivative through a Dimroth rearrangement reaction with 1-(4-nitrophenyl)-1H-1,2,3-triazole-4-carbaldehyde. This method can be used to obtain N-terminal-modified proteins via only two steps: 1) convenient preparation of a TA4C derivative with a functional group and 2) modification of the N terminus of the protein with the TA4C derivative.
中文翻译:

用于一步N末端蛋白质修饰的三唑甲醛试剂。
肽和蛋白质的位点特异性修饰是蛋白质工程的关键方面。我们开发了一种使用1H-1,2,3-三唑-4-甲醛(TA4C)衍生物修饰蛋白质N端的方法,该方法可以一步制备。用TA4C衍生物对生物活性肽和蛋白质进行N端特异性标记可在温和的反应条件下以优异的转化率进行(血管紧张素I:92%,核糖核酸酶A:90%)。该方法能够使各种功能分子(例如荧光团,生物素和连接至N末端三唑环的聚乙二醇)进行位点特异性缀合。此外,可以通过与1-(4-硝基苯基)-1H-1,2,3-三唑-4-甲醛的Dimroth重排反应将伯胺部分修饰的功能分子直接转化为TA4C衍生物。
更新日期:2020-01-16
中文翻译:

用于一步N末端蛋白质修饰的三唑甲醛试剂。
肽和蛋白质的位点特异性修饰是蛋白质工程的关键方面。我们开发了一种使用1H-1,2,3-三唑-4-甲醛(TA4C)衍生物修饰蛋白质N端的方法,该方法可以一步制备。用TA4C衍生物对生物活性肽和蛋白质进行N端特异性标记可在温和的反应条件下以优异的转化率进行(血管紧张素I:92%,核糖核酸酶A:90%)。该方法能够使各种功能分子(例如荧光团,生物素和连接至N末端三唑环的聚乙二醇)进行位点特异性缀合。此外,可以通过与1-(4-硝基苯基)-1H-1,2,3-三唑-4-甲醛的Dimroth重排反应将伯胺部分修饰的功能分子直接转化为TA4C衍生物。

























 京公网安备 11010802027423号
京公网安备 11010802027423号