当前位置:
X-MOL 学术
›
Blood Cancer J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ofatumumab maintenance prolongs progression-free survival in relapsed chronic lymphocytic leukemia: final analysis of the PROLONG study.
Blood Cancer Journal ( IF 12.8 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41408-019-0260-2 Marinus van Oers 1 , Lukas Smolej 2 , Mario Petrini 3 , Fritz Offner 4 , Sebastian Grosicki 5 , Mark-David Levin 6 , Jaclyn Davis 7 , Hiya Banerjee 7 , Tommaso Stefanelli 8 , Petra Hoever 8 , Christian Geisler 9
Blood Cancer Journal ( IF 12.8 ) Pub Date : 2019-12-04 , DOI: 10.1038/s41408-019-0260-2 Marinus van Oers 1 , Lukas Smolej 2 , Mario Petrini 3 , Fritz Offner 4 , Sebastian Grosicki 5 , Mark-David Levin 6 , Jaclyn Davis 7 , Hiya Banerjee 7 , Tommaso Stefanelli 8 , Petra Hoever 8 , Christian Geisler 9
Affiliation
|
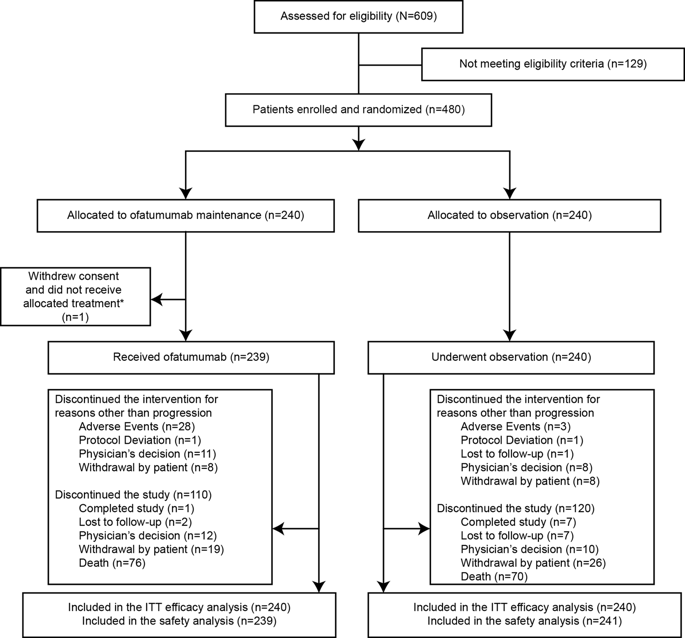
We report the final analysis of the PROLONG study on ofatumumab maintenance in relapsed chronic lymphocytic leukemia (CLL). In all, 480 patients with CLL in complete or partial remission after second- or third-line treatment were randomized 1:1 to ofatumumab (300 mg first week, followed by 1000 mg every 8 weeks for up to 2 years) or observation. Median follow-up duration was 40.9 months. Median progression-free survival was 34.2 and 16.9 months for ofatumumab and observation arms, respectively, (hazard ratio, 0.55 [95% confidence interval, 0.43-0.70]; P < 0.0001). Median time to next treatment for ofatumumab and observation arms, respectively, was 37.4 and 27.6 months (0.72 [0.57-0.91]; P = 0.0044). Overall survival was similar in both arms; median was not reached (0.99 [0.72-1.37]). Grade ≥ 3 adverse events occurred in 62% and 51% of patients in ofatumumab and observation arms, respectively, the most common being neutropenia (23% and 10%), pneumonia (13% and 12%) and febrile neutropenia (6% and 4%). Up to 60 days after the last treatment, four deaths were reported in the ofatumumab arm versus six in the observation arm, none considered related to ofatumumab. Ofatumumab maintenance significantly prolonged progression-free survival in patients with relapsed CLL and was well tolerated.
中文翻译:

Ofatumumab维持治疗可延长复发性慢性淋巴细胞白血病的无进展生存期:PROLONG研究的最终分析。
我们报告复发的慢性淋巴细胞性白血病(CLL)中ofatumumab维持的PROLONG研究的最终分析。总共将480例二线或三线治疗后完全缓解或部分缓解的CLL患者按1:1的比例随机分配至ofatumumab(第一周300 mg,随后每8周1000 mg,持续2年)或观察。中位随访时间为40.9个月。Ofatumumab和观察组的中位无进展生存期分别为34.2和16.9个月(危险比,0.55 [95%置信区间,0.43-0.70]; P <0.0001)。Ofatumumab和观察组下次治疗的中位时间分别为37.4和27.6个月(0.72 [0.57-0.91]; P = 0.0044)。两组的总生存率相似。中位数未达到(0.99 [0.72-1.37])。在ofatumumab和观察组中,分别有62%和51%的患者发生≥3级不良事件,最常见的是中性粒细胞减少症(23%和10%),肺炎(13%和12%)和高热性中性粒细胞减少症(6%和6%)。 4%)。在最后一次治疗后的60天之内,ofatumumab组报告了4例死亡,而观察组报告了6例死亡,均无与OFatumumab相关的报道。Ofatumumab的维持显着延长了CLL复发患者的无进展生存期,并且耐受性良好。
更新日期:2019-12-04
中文翻译:

Ofatumumab维持治疗可延长复发性慢性淋巴细胞白血病的无进展生存期:PROLONG研究的最终分析。
我们报告复发的慢性淋巴细胞性白血病(CLL)中ofatumumab维持的PROLONG研究的最终分析。总共将480例二线或三线治疗后完全缓解或部分缓解的CLL患者按1:1的比例随机分配至ofatumumab(第一周300 mg,随后每8周1000 mg,持续2年)或观察。中位随访时间为40.9个月。Ofatumumab和观察组的中位无进展生存期分别为34.2和16.9个月(危险比,0.55 [95%置信区间,0.43-0.70]; P <0.0001)。Ofatumumab和观察组下次治疗的中位时间分别为37.4和27.6个月(0.72 [0.57-0.91]; P = 0.0044)。两组的总生存率相似。中位数未达到(0.99 [0.72-1.37])。在ofatumumab和观察组中,分别有62%和51%的患者发生≥3级不良事件,最常见的是中性粒细胞减少症(23%和10%),肺炎(13%和12%)和高热性中性粒细胞减少症(6%和6%)。 4%)。在最后一次治疗后的60天之内,ofatumumab组报告了4例死亡,而观察组报告了6例死亡,均无与OFatumumab相关的报道。Ofatumumab的维持显着延长了CLL复发患者的无进展生存期,并且耐受性良好。



























 京公网安备 11010802027423号
京公网安备 11010802027423号