当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Structure-Activity Relationships for CYP4B1 Bioactivation of 4-Ipomeanol Congeners: Direct Correlation between Cytotoxicity and Trapped Reactive Intermediates.
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.chemrestox.9b00330 John P Kowalski 1 , Matthew G McDonald 1 , Dale Whittington 1 , Miklos Guttman 1 , Michele Scian 1 , Marco Girhard 2 , Helmut Hanenberg 3 , Constanze Wiek 4 , Allan E Rettie 1
Chemical Research in Toxicology ( IF 4.1 ) Pub Date : 2019-12-04 , DOI: 10.1021/acs.chemrestox.9b00330 John P Kowalski 1 , Matthew G McDonald 1 , Dale Whittington 1 , Miklos Guttman 1 , Michele Scian 1 , Marco Girhard 2 , Helmut Hanenberg 3 , Constanze Wiek 4 , Allan E Rettie 1
Affiliation
|
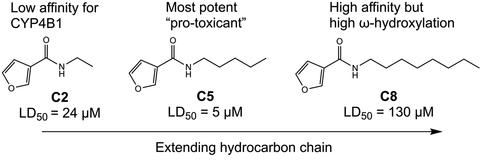
Cytochrome P450 4B1 (CYP4B1) has been explored as a candidate enzyme in suicide gene systems for its ability to bioactivate the natural product 4-ipomeanol (IPO) to a reactive species that causes cytotoxicity. However, metabolic limitations of IPO necessitate discovery of new "pro-toxicant" substrates for CYP4B1. In the present study, we examined a series of synthetically facile N-alkyl-3-furancarboxamides for cytotoxicity in HepG2 cells expressing CYP4B1. This compound series maintains the furan warhead of IPO while replacing its alcohol group with alkyl chains of varying length (C1-C8). Compounds with C3-C6 carbon chain lengths showed similar potency to IPO (LD50 ≈ 5 μM). Short chain analogs (<3 carbons) and long chain analogs (>6 carbons) exhibited reduced toxicity, resulting in a parabolic relationship between alkyl chain length and cytotoxicity. A similar parabolic relationship was observed between alkyl chain length and reactive intermediate formation upon trapping of the putative enedial as a stable pyrrole adduct in incubations with purified recombinant rabbit CYP4B1 and common physiological nucleophiles. These parabolic relationships reflect the lower affinity of shorter chain compounds for CYP4B1 and increased ω-hydroxylation of the longer chain compounds by the enzyme. Furthermore, modest time-dependent inhibition of CYP4B1 by N-pentyl-3-furancarboxamide was completely abolished when trapping agents were added, demonstrating escape of reactive intermediates from the enzyme after bioactivation. An insulated CYP4B1 active site may explain the rarely observed direct correlation between adduct formation and cell toxicity reported here.
中文翻译:

CYP4B1 4-Ipomeanol 同源物的生物活化的构效关系:细胞毒性和捕获的反应性中间体之间的直接相关性。
细胞色素 P450 4B1 (CYP4B1) 已被探索作为自杀基因系统中的候选酶,因为它能够将天然产物 4-ipomeanol (IPO) 生物活化为引起细胞毒性的反应性物质。然而,IPO 的代谢限制需要为 CYP4B1 发现新的“促毒”底物。在本研究中,我们检查了一系列易于合成的 N-烷基-3-呋喃甲酰胺在表达 CYP4B1 的 HepG2 细胞中的细胞毒性。该化合物系列保留了IPO的呋喃弹头,同时用不同长度的烷基链(C1-C8)取代了其醇基。具有 C3-C6 碳链长度的化合物显示出与 IPO 相似的效力(LD50 ≈ 5 μM)。短链类似物(<3 个碳)和长链类似物(>6 个碳)表现出降低的毒性,导致烷基链长度和细胞毒性之间的抛物线关系。在与纯化的重组兔 CYP4B1 和常见的生理亲核试剂一起孵育时,在作为稳定的吡咯加合物的推定的烯二醇被捕获后,在烷基链长度和反应性中间体形成之间观察到类似的抛物线关系。这些抛物线关系反映了较短链化合物对 CYP4B1 的亲和力较低,以及酶对较长链化合物的 ω-羟基化增加。此外,当添加捕集剂时,N-戊基-3-呋喃甲酰胺对 CYP4B1 的适度时间依赖性抑制完全消除,表明生物活化后反应性中间体从酶中逃逸。
更新日期:2019-12-04
中文翻译:

CYP4B1 4-Ipomeanol 同源物的生物活化的构效关系:细胞毒性和捕获的反应性中间体之间的直接相关性。
细胞色素 P450 4B1 (CYP4B1) 已被探索作为自杀基因系统中的候选酶,因为它能够将天然产物 4-ipomeanol (IPO) 生物活化为引起细胞毒性的反应性物质。然而,IPO 的代谢限制需要为 CYP4B1 发现新的“促毒”底物。在本研究中,我们检查了一系列易于合成的 N-烷基-3-呋喃甲酰胺在表达 CYP4B1 的 HepG2 细胞中的细胞毒性。该化合物系列保留了IPO的呋喃弹头,同时用不同长度的烷基链(C1-C8)取代了其醇基。具有 C3-C6 碳链长度的化合物显示出与 IPO 相似的效力(LD50 ≈ 5 μM)。短链类似物(<3 个碳)和长链类似物(>6 个碳)表现出降低的毒性,导致烷基链长度和细胞毒性之间的抛物线关系。在与纯化的重组兔 CYP4B1 和常见的生理亲核试剂一起孵育时,在作为稳定的吡咯加合物的推定的烯二醇被捕获后,在烷基链长度和反应性中间体形成之间观察到类似的抛物线关系。这些抛物线关系反映了较短链化合物对 CYP4B1 的亲和力较低,以及酶对较长链化合物的 ω-羟基化增加。此外,当添加捕集剂时,N-戊基-3-呋喃甲酰胺对 CYP4B1 的适度时间依赖性抑制完全消除,表明生物活化后反应性中间体从酶中逃逸。


























 京公网安备 11010802027423号
京公网安备 11010802027423号