当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A synthetic route to 1,4-disubstituted tetrahydro-β-carbolines and tetrahydropyranoindoles via ring-opening/Pictet-Spengler reaction of aziridines and epoxides with indoles/aldehydes.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02098e Imtiyaz Ahmad Wani 1 , Gaurav Goswami , Sahid Sk , Abhijit Mal , Masthanvali Sayyad , Manas K Ghorai
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2020-01-02 , DOI: 10.1039/c9ob02098e Imtiyaz Ahmad Wani 1 , Gaurav Goswami , Sahid Sk , Abhijit Mal , Masthanvali Sayyad , Manas K Ghorai
Affiliation
|
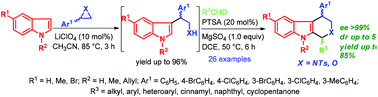
A simple and efficient synthetic route to various 1,4-disubstituted tetrahydro-β-carbolines and tetrahydropyrano[3,4-b]indoles in high yields and stereoselectivity via LiClO4-catalyzed SN2-type ring opening of aziridines and epoxides with indoles followed by p-toluenesulfonic acid (PTSA) catalyzed Pictet-Spengler reaction is described.
中文翻译:

通过氮丙啶和环氧化物与吲哚/醛的开环/ Pictet-Spengler反应合成1,4-二取代的四氢-β-咔啉和四氢吡喃并吲哚的合成路线。
一种简单有效的合成路线,可通过LiClO4催化的氮丙啶和环氧化物的SN2型开环与吲哚,然后是吲哚,高产率和立体选择性地合成各种1,4-二取代的四氢-β-咔啉和四氢吡喃并[3,4-b]吲哚描述了对甲苯磺酸(PTSA)催化的Pictet-Spengler反应。
更新日期:2020-01-02
中文翻译:

通过氮丙啶和环氧化物与吲哚/醛的开环/ Pictet-Spengler反应合成1,4-二取代的四氢-β-咔啉和四氢吡喃并吲哚的合成路线。
一种简单有效的合成路线,可通过LiClO4催化的氮丙啶和环氧化物的SN2型开环与吲哚,然后是吲哚,高产率和立体选择性地合成各种1,4-二取代的四氢-β-咔啉和四氢吡喃并[3,4-b]吲哚描述了对甲苯磺酸(PTSA)催化的Pictet-Spengler反应。

























 京公网安备 11010802027423号
京公网安备 11010802027423号