当前位置:
X-MOL 学术
›
Lancet Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study
The Lancet Neurology ( IF 48.0 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30394-1 Katrina M Moore 1 , Jennifer Nicholas 2 , Murray Grossman 3 , Corey T McMillan 3 , David J Irwin 3 , Lauren Massimo 3 , Vivianna M Van Deerlin 3 , Jason D Warren 1 , Nick C Fox 1 , Martin N Rossor 1 , Simon Mead 4 , Martina Bocchetta 1 , Bradley F Boeve 5 , David S Knopman 5 , Neill R Graff-Radford 6 , Leah K Forsberg 5 , Rosa Rademakers 7 , Zbigniew K Wszolek 6 , John C van Swieten 8 , Lize C Jiskoot 8 , Lieke H Meeter 8 , Elise Gp Dopper 8 , Janne M Papma 8 , Julie S Snowden 9 , Jennifer Saxon 9 , Matthew Jones 9 , Stuart Pickering-Brown 9 , Isabelle Le Ber 10 , Agnès Camuzat 10 , Alexis Brice 10 , Paola Caroppo 10 , Roberta Ghidoni 11 , Michela Pievani 12 , Luisa Benussi 11 , Giuliano Binetti 11 , Bradford C Dickerson 13 , Diane Lucente 13 , Samantha Krivensky 13 , Caroline Graff 14 , Linn Öijerstedt 14 , Marie Fallström 14 , Håkan Thonberg 14 , Nupur Ghoshal 15 , John C Morris 15 , Barbara Borroni 16 , Alberto Benussi 16 , Alessandro Padovani 16 , Daniela Galimberti 17 , Elio Scarpini 18 , Giorgio G Fumagalli 19 , Ian R Mackenzie 20 , Ging-Yuek R Hsiung 20 , Pheth Sengdy 20 , Adam L Boxer 21 , Howie Rosen 21 , Joanne B Taylor 21 , Matthis Synofzik 22 , Carlo Wilke 22 , Patricia Sulzer 22 , John R Hodges 23 , Glenda Halliday 23 , John Kwok 23 , Raquel Sanchez-Valle 24 , Albert Lladó 24 , Sergi Borrego-Ecija 24 , Isabel Santana 25 , Maria Rosário Almeida 26 , Miguel Tábuas-Pereira 27 , Fermin Moreno 28 , Myriam Barandiaran 28 , Begoña Indakoetxea 28 , Johannes Levin 29 , Adrian Danek 30 , James B Rowe 31 , Thomas E Cope 31 , Markus Otto 32 , Sarah Anderl-Straub 32 , Alexandre de Mendonça 33 , Carolina Maruta 33 , Mario Masellis 34 , Sandra E Black 34 , Philippe Couratier 35 , Geraldine Lautrette 35 , Edward D Huey 36 , Sandro Sorbi 37 , Benedetta Nacmias 38 , Robert Laforce 39 , Marie-Pier L Tremblay 39 , Rik Vandenberghe 40 , Philip Van Damme 41 , Emily J Rogalski 42 , Sandra Weintraub 42 , Alexander Gerhard 43 , Chiadi U Onyike 44 , Simon Ducharme 45 , Sokratis G Papageorgiou 46 , Adeline Su Lyn Ng 47 , Amy Brodtmann 48 , Elizabeth Finger 49 , Rita Guerreiro 50 , Jose Bras 50 , Jonathan D Rohrer 1 ,
The Lancet Neurology ( IF 48.0 ) Pub Date : 2020-02-01 , DOI: 10.1016/s1474-4422(19)30394-1 Katrina M Moore 1 , Jennifer Nicholas 2 , Murray Grossman 3 , Corey T McMillan 3 , David J Irwin 3 , Lauren Massimo 3 , Vivianna M Van Deerlin 3 , Jason D Warren 1 , Nick C Fox 1 , Martin N Rossor 1 , Simon Mead 4 , Martina Bocchetta 1 , Bradley F Boeve 5 , David S Knopman 5 , Neill R Graff-Radford 6 , Leah K Forsberg 5 , Rosa Rademakers 7 , Zbigniew K Wszolek 6 , John C van Swieten 8 , Lize C Jiskoot 8 , Lieke H Meeter 8 , Elise Gp Dopper 8 , Janne M Papma 8 , Julie S Snowden 9 , Jennifer Saxon 9 , Matthew Jones 9 , Stuart Pickering-Brown 9 , Isabelle Le Ber 10 , Agnès Camuzat 10 , Alexis Brice 10 , Paola Caroppo 10 , Roberta Ghidoni 11 , Michela Pievani 12 , Luisa Benussi 11 , Giuliano Binetti 11 , Bradford C Dickerson 13 , Diane Lucente 13 , Samantha Krivensky 13 , Caroline Graff 14 , Linn Öijerstedt 14 , Marie Fallström 14 , Håkan Thonberg 14 , Nupur Ghoshal 15 , John C Morris 15 , Barbara Borroni 16 , Alberto Benussi 16 , Alessandro Padovani 16 , Daniela Galimberti 17 , Elio Scarpini 18 , Giorgio G Fumagalli 19 , Ian R Mackenzie 20 , Ging-Yuek R Hsiung 20 , Pheth Sengdy 20 , Adam L Boxer 21 , Howie Rosen 21 , Joanne B Taylor 21 , Matthis Synofzik 22 , Carlo Wilke 22 , Patricia Sulzer 22 , John R Hodges 23 , Glenda Halliday 23 , John Kwok 23 , Raquel Sanchez-Valle 24 , Albert Lladó 24 , Sergi Borrego-Ecija 24 , Isabel Santana 25 , Maria Rosário Almeida 26 , Miguel Tábuas-Pereira 27 , Fermin Moreno 28 , Myriam Barandiaran 28 , Begoña Indakoetxea 28 , Johannes Levin 29 , Adrian Danek 30 , James B Rowe 31 , Thomas E Cope 31 , Markus Otto 32 , Sarah Anderl-Straub 32 , Alexandre de Mendonça 33 , Carolina Maruta 33 , Mario Masellis 34 , Sandra E Black 34 , Philippe Couratier 35 , Geraldine Lautrette 35 , Edward D Huey 36 , Sandro Sorbi 37 , Benedetta Nacmias 38 , Robert Laforce 39 , Marie-Pier L Tremblay 39 , Rik Vandenberghe 40 , Philip Van Damme 41 , Emily J Rogalski 42 , Sandra Weintraub 42 , Alexander Gerhard 43 , Chiadi U Onyike 44 , Simon Ducharme 45 , Sokratis G Papageorgiou 46 , Adeline Su Lyn Ng 47 , Amy Brodtmann 48 , Elizabeth Finger 49 , Rita Guerreiro 50 , Jose Bras 50 , Jonathan D Rohrer 1 ,
Affiliation
|
BACKGROUND
Frontotemporal dementia is a heterogenous neurodegenerative disorder, with about a third of cases being genetic. Most of this genetic component is accounted for by mutations in GRN, MAPT, and C9orf72. In this study, we aimed to complement previous phenotypic studies by doing an international study of age at symptom onset, age at death, and disease duration in individuals with mutations in GRN, MAPT, and C9orf72. METHODS
In this international, retrospective cohort study, we collected data on age at symptom onset, age at death, and disease duration for patients with pathogenic mutations in the GRN and MAPT genes and pathological expansions in the C9orf72 gene through the Frontotemporal Dementia Prevention Initiative and from published papers. We used mixed effects models to explore differences in age at onset, age at death, and disease duration between genetic groups and individual mutations. We also assessed correlations between the age at onset and at death of each individual and the age at onset and at death of their parents and the mean age at onset and at death of their family members. Lastly, we used mixed effects models to investigate the extent to which variability in age at onset and at death could be accounted for by family membership and the specific mutation carried. FINDINGS
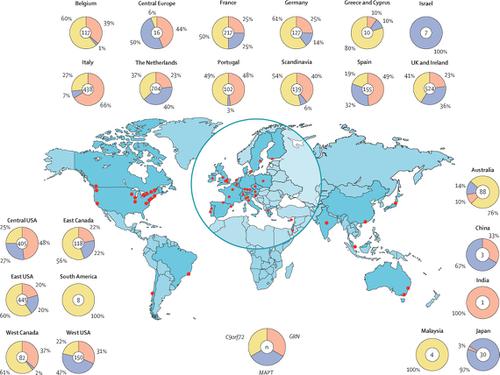
Data were available from 3403 individuals from 1492 families: 1433 with C9orf72 expansions (755 families), 1179 with GRN mutations (483 families, 130 different mutations), and 791 with MAPT mutations (254 families, 67 different mutations). Mean age at symptom onset and at death was 49·5 years (SD 10·0; onset) and 58·5 years (11·3; death) in the MAPT group, 58·2 years (9·8; onset) and 65·3 years (10·9; death) in the C9orf72 group, and 61·3 years (8·8; onset) and 68·8 years (9·7; death) in the GRN group. Mean disease duration was 6·4 years (SD 4·9) in the C9orf72 group, 7·1 years (3·9) in the GRN group, and 9·3 years (6·4) in the MAPT group. Individual age at onset and at death was significantly correlated with both parental age at onset and at death and with mean family age at onset and at death in all three groups, with a stronger correlation observed in the MAPT group (r=0·45 between individual and parental age at onset, r=0·63 between individual and mean family age at onset, r=0·58 between individual and parental age at death, and r=0·69 between individual and mean family age at death) than in either the C9orf72 group (r=0·32 individual and parental age at onset, r=0·36 individual and mean family age at onset, r=0·38 individual and parental age at death, and r=0·40 individual and mean family age at death) or the GRN group (r=0·22 individual and parental age at onset, r=0·18 individual and mean family age at onset, r=0·22 individual and parental age at death, and r=0·32 individual and mean family age at death). Modelling showed that the variability in age at onset and at death in the MAPT group was explained partly by the specific mutation (48%, 95% CI 35-62, for age at onset; 61%, 47-73, for age at death), and even more by family membership (66%, 56-75, for age at onset; 74%, 65-82, for age at death). In the GRN group, only 2% (0-10) of the variability of age at onset and 9% (3-21) of that of age of death was explained by the specific mutation, whereas 14% (9-22) of the variability of age at onset and 20% (12-30) of that of age at death was explained by family membership. In the C9orf72 group, family membership explained 17% (11-26) of the variability of age at onset and 19% (12-29) of that of age at death. INTERPRETATION
Our study showed that age at symptom onset and at death of people with genetic frontotemporal dementia is influenced by genetic group and, particularly for MAPT mutations, by the specific mutation carried and by family membership. Although estimation of age at onset will be an important factor in future pre-symptomatic therapeutic trials for all three genetic groups, our study suggests that data from other members of the family will be particularly helpful only for individuals with MAPT mutations. Further work in identifying both genetic and environmental factors that modify phenotype in all groups will be important to improve such estimates. FUNDING
UK Medical Research Council, National Institute for Health Research, and Alzheimer's Society.
中文翻译:

遗传性额颞叶痴呆的发病年龄、死亡和病程:一项国际回顾性队列研究
背景技术额颞叶痴呆是一种异源性神经退行性疾病,大约三分之一的病例是遗传的。这种遗传成分的大部分是由 GRN、MAPT 和 C9orf72 中的突变造成的。在这项研究中,我们旨在通过对 GRN、MAPT 和 C9orf72 突变个体的症状发作年龄、死亡年龄和疾病持续时间进行国际研究来补充先前的表型研究。方法 在这项国际回顾性队列研究中,我们通过额颞叶痴呆预防倡议和从已发表的论文中。我们使用混合效应模型来探索发病年龄、死亡年龄、遗传群体和个体突变之间的疾病持续时间。我们还评估了每个人的发病年龄和死亡年龄与其父母的发病年龄和死亡年龄及其家庭成员的平均发病年龄和死亡年龄之间的相关性。最后,我们使用混合效应模型来研究发病年龄和死亡年龄的变异性在多大程度上可以由家庭成员和携带的特定突变来解释。结果 数据来自 1492 个家族的 3403 名个体:1433 名具有 C9orf72 扩展(755 个家族),1179 名具有 GRN 突变(483 个家族,130 个不同的突变),791 名具有 MAPT 突变(254 个家族,67 个不同的突变)。MAPT 组出现症状和死亡的平均年龄分别为 49·5 岁(SD 10·0;发病)和 58·5 岁(11·3;死亡),58·2 岁(9·8;C9orf72 组发病)和 65·3 年(10·9;死亡),GRN 组分别为 61·3 年(8·8;发病)和 68·8 年(9·7;死亡)。C9orf72组的平均病程为6·4年(SD 4·9),GRN组为7·1年(3·9),MAPT组为9·3年(6·4)。在所有三组中,个体发病和死亡年龄均与父母发病和死亡年龄以及平均家庭发病和死亡年龄显着相关,在 MAPT 组中观察到更强的相关性(r=0·45 之间个人和父母发病年龄,r=0·63 个人和平均家庭发病年龄,r=0·58 个人和父母死亡年龄,r=0·69 个人和平均家庭死亡年龄)在 C9orf72 组中(r=0·32 个人和父母发病年龄,r=0·36 个人和平均家庭发病年龄,r=0·38 个体和父母死亡年龄,r=0·40 个体和平均家庭死亡年龄)或 GRN 组(r=0·22 个体和父母发病年龄,r=0·18 个体和发病时的平均家庭年龄,r=0·22 个人和父母死亡时的年龄,r=0·32 个人和死亡时的平均家庭年龄)。建模显示,MAPT 组发病年龄和死亡年龄的差异部分由特定突变解释(发病年龄为 48%,95% CI 35-62;死亡年龄为 61%,47-73 ),甚至更多的是家庭成员(发病年龄为 66%,56-75 岁;死亡年龄为 74%,65-82 岁)。在 GRN 组中,只有 2% (0-10) 的发病年龄变异性和 9% (3-21) 的死亡年龄变异是由特定突变解释的,而 14% (9-22) 的发病年龄变异性和 20% (12-30) 的死亡年龄变异性由家庭成员解释。在 C9orf72 组中,家庭成员解释了 17% (11-26) 的发病年龄变异性和 19% (12-29) 的死亡年龄变异性。解释 我们的研究表明,遗传性额颞叶痴呆患者出现症状和死亡时的年龄受遗传群体的影响,特别是对于 MAPT 突变,受携带的特定突变和家庭成员的影响。尽管估计发病年龄将是未来所有三个基因组的症状前治疗试验中的一个重要因素,但我们的研究表明,来自家庭其他成员的数据将仅对具有 MAPT 突变的个体特别有用。在确定所有组中改变表型的遗传和环境因素方面的进一步工作对于改进此类估计非常重要。资助英国医学研究委员会、国家健康研究所和阿尔茨海默氏症协会。
更新日期:2020-02-01
中文翻译:

遗传性额颞叶痴呆的发病年龄、死亡和病程:一项国际回顾性队列研究
背景技术额颞叶痴呆是一种异源性神经退行性疾病,大约三分之一的病例是遗传的。这种遗传成分的大部分是由 GRN、MAPT 和 C9orf72 中的突变造成的。在这项研究中,我们旨在通过对 GRN、MAPT 和 C9orf72 突变个体的症状发作年龄、死亡年龄和疾病持续时间进行国际研究来补充先前的表型研究。方法 在这项国际回顾性队列研究中,我们通过额颞叶痴呆预防倡议和从已发表的论文中。我们使用混合效应模型来探索发病年龄、死亡年龄、遗传群体和个体突变之间的疾病持续时间。我们还评估了每个人的发病年龄和死亡年龄与其父母的发病年龄和死亡年龄及其家庭成员的平均发病年龄和死亡年龄之间的相关性。最后,我们使用混合效应模型来研究发病年龄和死亡年龄的变异性在多大程度上可以由家庭成员和携带的特定突变来解释。结果 数据来自 1492 个家族的 3403 名个体:1433 名具有 C9orf72 扩展(755 个家族),1179 名具有 GRN 突变(483 个家族,130 个不同的突变),791 名具有 MAPT 突变(254 个家族,67 个不同的突变)。MAPT 组出现症状和死亡的平均年龄分别为 49·5 岁(SD 10·0;发病)和 58·5 岁(11·3;死亡),58·2 岁(9·8;C9orf72 组发病)和 65·3 年(10·9;死亡),GRN 组分别为 61·3 年(8·8;发病)和 68·8 年(9·7;死亡)。C9orf72组的平均病程为6·4年(SD 4·9),GRN组为7·1年(3·9),MAPT组为9·3年(6·4)。在所有三组中,个体发病和死亡年龄均与父母发病和死亡年龄以及平均家庭发病和死亡年龄显着相关,在 MAPT 组中观察到更强的相关性(r=0·45 之间个人和父母发病年龄,r=0·63 个人和平均家庭发病年龄,r=0·58 个人和父母死亡年龄,r=0·69 个人和平均家庭死亡年龄)在 C9orf72 组中(r=0·32 个人和父母发病年龄,r=0·36 个人和平均家庭发病年龄,r=0·38 个体和父母死亡年龄,r=0·40 个体和平均家庭死亡年龄)或 GRN 组(r=0·22 个体和父母发病年龄,r=0·18 个体和发病时的平均家庭年龄,r=0·22 个人和父母死亡时的年龄,r=0·32 个人和死亡时的平均家庭年龄)。建模显示,MAPT 组发病年龄和死亡年龄的差异部分由特定突变解释(发病年龄为 48%,95% CI 35-62;死亡年龄为 61%,47-73 ),甚至更多的是家庭成员(发病年龄为 66%,56-75 岁;死亡年龄为 74%,65-82 岁)。在 GRN 组中,只有 2% (0-10) 的发病年龄变异性和 9% (3-21) 的死亡年龄变异是由特定突变解释的,而 14% (9-22) 的发病年龄变异性和 20% (12-30) 的死亡年龄变异性由家庭成员解释。在 C9orf72 组中,家庭成员解释了 17% (11-26) 的发病年龄变异性和 19% (12-29) 的死亡年龄变异性。解释 我们的研究表明,遗传性额颞叶痴呆患者出现症状和死亡时的年龄受遗传群体的影响,特别是对于 MAPT 突变,受携带的特定突变和家庭成员的影响。尽管估计发病年龄将是未来所有三个基因组的症状前治疗试验中的一个重要因素,但我们的研究表明,来自家庭其他成员的数据将仅对具有 MAPT 突变的个体特别有用。在确定所有组中改变表型的遗传和环境因素方面的进一步工作对于改进此类估计非常重要。资助英国医学研究委员会、国家健康研究所和阿尔茨海默氏症协会。



























 京公网安备 11010802027423号
京公网安备 11010802027423号