当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Three stable dinuclear [M2(OH)0.5(NO3)0.5(RCOO)2(RN)4] (M = Cu, Ni) based metal–organic frameworks with high CO2 adsorption and selective separation for O2/N2 and C3H8/CH4
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/03 , DOI: 10.1039/c9qi01220f Liang Kan 1, 2, 3, 4, 5 , Lan Li 1, 2, 3, 4, 5 , Guanghua Li 1, 2, 3, 4, 5 , Lirong Zhang 1, 2, 3, 4, 5 , Yunling Liu 1, 2, 3, 4, 5
Inorganic Chemistry Frontiers ( IF 7 ) Pub Date : 2019/12/03 , DOI: 10.1039/c9qi01220f Liang Kan 1, 2, 3, 4, 5 , Lan Li 1, 2, 3, 4, 5 , Guanghua Li 1, 2, 3, 4, 5 , Lirong Zhang 1, 2, 3, 4, 5 , Yunling Liu 1, 2, 3, 4, 5
Affiliation
|
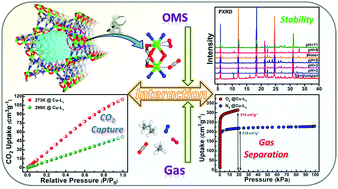
Three highly porous MOFs with dinuclear [M2(OH)0.5(NO3)0.5(RCOO)2(RN)4] SBUs (secondary building units), [Cu4(L1)4(OH)(NO3)]·2(NO3)·4DMF (compound Cu-L1, HL1 = 5-imidazol-1-yl-nicotinic acid, DMF = N,N-dimethylformamide), [Ni4(L1)4(OH)(NO3)3(DMF)2]·3DMF·4H2O (compound Ni-L1) and [Ni4(L2)4(OH)(NO3)3(DMF)2]·3DMF (compound Ni-L2, HL2 = 5-[1,2,4]triazol-1-yl-nicotinic acid), have been solvothermally constructed using a heterofunctional ligand motif with carboxylate and azolate groups. All three compounds show high thermal and chemical stability, not only in water but also in acidic and basic solutions (pH = 2–11). Due to the presence of accessible open metal sites (OMSs), compounds Cu-L1, Ni-L1, and Ni-L2 exhibit significant ability to capture CO2 (114.8, 88.3 and 93.7 cm3 g−1, respectively, under 1 bar at 273 K). Interestingly, gas adsorption results show that all three compounds exhibit high selectivity toward O2 over N2 at 77 K and C3H8 over CH4 at 298 K, which indicates that the three materials have potential for gas separation applications.
中文翻译:

三种稳定的双核[M2(OH)0.5(NO3)0.5(RCOO)2(RN)4](M = Cu,Ni)基金属-有机骨架,具有高的CO2吸附能力以及对O2 / N2和C3H8 / CH4的选择性分离
具有双核[M 2(OH)0.5(NO 3)0.5(RCOO)2(RN)4 ] SBU(二级结构单元),[Cu 4(L 1)4(OH)(NO 3)]的三个高度多孔的MOF。·2(NO 3)·4DMF(化合物Cu-L 1,HL 1 = 5-咪唑-1-基-烟酸,DMF = N,N-二甲基甲酰胺),[Ni 4(L 1)4(OH)( NO 3)3(DMF)2 ]·3DMF·4H 2O(化合物Ni-L 1)和[Ni 4(L 2)4(OH)(NO 3)3(DMF)2 ]·3DMF(化合物Ni-L 2,HL 2 = 5- [1,2,4 [三唑-1-基-烟酸],已使用具有羧酸根和偶氮根基团的杂功能配体基序进行溶剂热构建。这三种化合物不仅在水中而且在酸性和碱性溶液(pH = 2-11)中均显示出高的热稳定性和化学稳定性。由于存在可访问的开放金属位点(OMS),因此化合物Cu-L 1,Ni-L 1和Ni-L 2表现出显着的捕获CO 2的能力(在273 K下1 bar下分别为114.8、88.3和93.7 cm 3 g -1)。有趣的是,气体吸附结果表明,这三种化合物对77 K的N 2的O 2和对298 K的CH 4的C 3 H 8都有很高的选择性,这表明这三种材料具有用于气体分离的潜力。
更新日期:2020-02-13
中文翻译:

三种稳定的双核[M2(OH)0.5(NO3)0.5(RCOO)2(RN)4](M = Cu,Ni)基金属-有机骨架,具有高的CO2吸附能力以及对O2 / N2和C3H8 / CH4的选择性分离
具有双核[M 2(OH)0.5(NO 3)0.5(RCOO)2(RN)4 ] SBU(二级结构单元),[Cu 4(L 1)4(OH)(NO 3)]的三个高度多孔的MOF。·2(NO 3)·4DMF(化合物Cu-L 1,HL 1 = 5-咪唑-1-基-烟酸,DMF = N,N-二甲基甲酰胺),[Ni 4(L 1)4(OH)( NO 3)3(DMF)2 ]·3DMF·4H 2O(化合物Ni-L 1)和[Ni 4(L 2)4(OH)(NO 3)3(DMF)2 ]·3DMF(化合物Ni-L 2,HL 2 = 5- [1,2,4 [三唑-1-基-烟酸],已使用具有羧酸根和偶氮根基团的杂功能配体基序进行溶剂热构建。这三种化合物不仅在水中而且在酸性和碱性溶液(pH = 2-11)中均显示出高的热稳定性和化学稳定性。由于存在可访问的开放金属位点(OMS),因此化合物Cu-L 1,Ni-L 1和Ni-L 2表现出显着的捕获CO 2的能力(在273 K下1 bar下分别为114.8、88.3和93.7 cm 3 g -1)。有趣的是,气体吸附结果表明,这三种化合物对77 K的N 2的O 2和对298 K的CH 4的C 3 H 8都有很高的选择性,这表明这三种材料具有用于气体分离的潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号