当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Asymmetric redox-neutral radical cyclization catalysed by flavin-dependent 'ene'-reductases.
Nature Chemistry ( IF 21.8 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41557-019-0370-2 Michael J Black 1 , Kyle F Biegasiewicz 1 , Andrew J Meichan 1 , Daniel G Oblinsky 1 , Bryan Kudisch 1 , Gregory D Scholes 1 , Todd K Hyster 1
Nature Chemistry ( IF 21.8 ) Pub Date : 2019-12-02 , DOI: 10.1038/s41557-019-0370-2 Michael J Black 1 , Kyle F Biegasiewicz 1 , Andrew J Meichan 1 , Daniel G Oblinsky 1 , Bryan Kudisch 1 , Gregory D Scholes 1 , Todd K Hyster 1
Affiliation
|
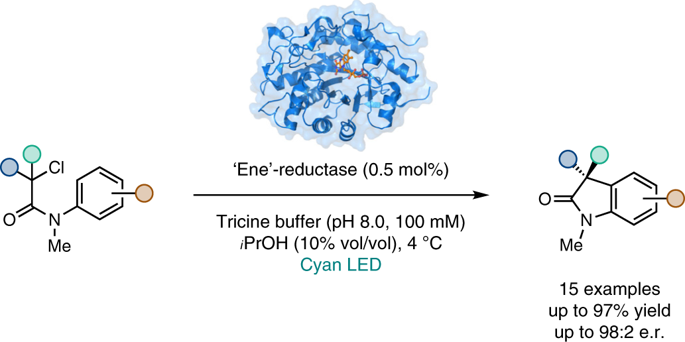
Flavin-dependent 'ene'-reductases (EREDs) are exquisite catalysts for effecting stereoselective reductions. Although these reactions typically proceed through a hydride transfer mechanism, we recently found that EREDs can also catalyse reductive dehalogenations and cyclizations via single electron transfer mechanisms. Here, we demonstrate that these enzymes can catalyse redox-neutral radical cyclizations to produce enantioenriched oxindoles from α-haloamides. This transformation is a C-C bond-forming reaction currently unknown in nature and one for which there are no catalytic asymmetric examples. Mechanistic studies indicate the reaction proceeds via the flavin semiquinone/quinone redox couple, where ground-state flavin semiquinone provides the electron for substrate reduction and flavin quinone oxidizes the vinylogous α-amido radical formed after cyclization. This mechanistic manifold was previously unknown for this enzyme family, highlighting the versatility of EREDs in asymmetric synthesis.
中文翻译:

由黄素依赖性“烯”还原酶催化的不对称氧化还原中性自由基环化。
黄素依赖性“烯”还原酶 (ERED) 是用于实现立体选择性还原的精致催化剂。尽管这些反应通常通过氢化物转移机制进行,但我们最近发现 ERED 也可以通过单电子转移机制催化还原性脱卤和环化。在这里,我们证明这些酶可以催化氧化还原中性自由基环化,以从 α-卤代酰胺生产对映体富集的羟吲哚。这种转变是目前在自然界中未知的 CC 键形成反应,并且没有催化不对称的例子。机理研究表明反应通过黄素半醌/醌氧化还原对进行,其中基态黄素半醌为底物还原提供电子,黄素醌氧化环化后形成的乙烯基α-酰胺基。这种酶家族以前不知道这种机制流形,突出了 ERED 在不对称合成中的多功能性。
更新日期:2019-12-02
中文翻译:

由黄素依赖性“烯”还原酶催化的不对称氧化还原中性自由基环化。
黄素依赖性“烯”还原酶 (ERED) 是用于实现立体选择性还原的精致催化剂。尽管这些反应通常通过氢化物转移机制进行,但我们最近发现 ERED 也可以通过单电子转移机制催化还原性脱卤和环化。在这里,我们证明这些酶可以催化氧化还原中性自由基环化,以从 α-卤代酰胺生产对映体富集的羟吲哚。这种转变是目前在自然界中未知的 CC 键形成反应,并且没有催化不对称的例子。机理研究表明反应通过黄素半醌/醌氧化还原对进行,其中基态黄素半醌为底物还原提供电子,黄素醌氧化环化后形成的乙烯基α-酰胺基。这种酶家族以前不知道这种机制流形,突出了 ERED 在不对称合成中的多功能性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号