当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Diastereoselective construction of carbazole-based spirooxindoles via the Levy three-component reaction.
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-12-18 , DOI: 10.1039/c9ob02013f Shao-Cong Zhan 1 , Jing Sun , Ru-Zhang Liu , Chao-Guo Yan
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2019-12-18 , DOI: 10.1039/c9ob02013f Shao-Cong Zhan 1 , Jing Sun , Ru-Zhang Liu , Chao-Guo Yan
Affiliation
|
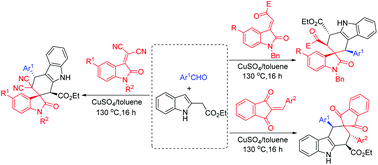
The CuSO4 catalyzed three-component reaction of indole-2-acetate, aromatic aldehydes and 3-methyleneoxindoles in toluene at 130 °C afforded polysubstituted spiro[carbazole-3,3'-indolines] in good yields and with high diastereoselectivity. When isatylidene malononitriles were used as dienophiles, regio-isomeric spiro[carbazole-2,3'-indolines] were selectively obtained. A similar three-component reaction with 2-arylidene-1,3-indanediones resulted in polysubstituted spiro[carbazole-3,2'-indenes] in satisfactory yields and with high diastereoselectivity. The stereochemistry of the diastereoisomers of the spiro compounds was clearly elucidated by analysis of NMR spectra and determination of fourteen single crystal structures. The reaction mechanism included formation of reactive 2,3-dimethyleneindoline and a sequential Diels-Alder reaction.
中文翻译:

通过Levy三组分反应非对映选择性构建咔唑基螺硫辛酯。
CuSO4在130°C下催化甲苯中的2-吲哚乙酸酯,芳族醛和3-亚甲基吲哚的三组分反应,得到的多取代螺环[咔唑-3,3'-二氢吲哚]的收率高,非对映选择性高。当异亚丙基丙二腈用作亲二烯体时,选择性地获得了区域异构的螺[咔唑-2,3'-二氢吲哚]。与2-亚芳基-1,3-茚满二酮的相似的三组分反应以令人满意的产率和高非对映选择性产生多取代的螺[咔唑-3,2'-茚]。螺环化合物的非对映异构体的立体化学通过NMR光谱分析和14个单晶结构的确定得以清楚地阐明。反应机理包括反应性2,3-二亚甲基二氢吲哚的形成和顺序的Diels-Alder反应。
更新日期:2020-01-15
中文翻译:

通过Levy三组分反应非对映选择性构建咔唑基螺硫辛酯。
CuSO4在130°C下催化甲苯中的2-吲哚乙酸酯,芳族醛和3-亚甲基吲哚的三组分反应,得到的多取代螺环[咔唑-3,3'-二氢吲哚]的收率高,非对映选择性高。当异亚丙基丙二腈用作亲二烯体时,选择性地获得了区域异构的螺[咔唑-2,3'-二氢吲哚]。与2-亚芳基-1,3-茚满二酮的相似的三组分反应以令人满意的产率和高非对映选择性产生多取代的螺[咔唑-3,2'-茚]。螺环化合物的非对映异构体的立体化学通过NMR光谱分析和14个单晶结构的确定得以清楚地阐明。反应机理包括反应性2,3-二亚甲基二氢吲哚的形成和顺序的Diels-Alder反应。


























 京公网安备 11010802027423号
京公网安备 11010802027423号