当前位置:
X-MOL 学术
›
JAMA Neurol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Fenfluramine for Treatment-Resistant Seizures in Patients With Dravet Syndrome Receiving Stiripentol-Inclusive Regimens: A Randomized Clinical Trial.
JAMA Neurology ( IF 29.0 ) Pub Date : 2019-12-02 , DOI: 10.1001/jamaneurol.2019.4113 Rima Nabbout 1 , Arun Mistry 2 , Sameer Zuberi 3 , Nathalie Villeneuve 4 , Antonio Gil-Nagel 5 , Rocio Sanchez-Carpintero 6 , Ulrich Stephani 7 , Linda Laux 8 , Elaine Wirrell 9 , Kelly Knupp 10 , Catherine Chiron 11 , Gail Farfel 2 , Bradley S Galer 2 , Glenn Morrison 2 , Michael Lock 2 , Anupam Agarwal 2 , Stéphane Auvin 12 ,
JAMA Neurology ( IF 29.0 ) Pub Date : 2019-12-02 , DOI: 10.1001/jamaneurol.2019.4113 Rima Nabbout 1 , Arun Mistry 2 , Sameer Zuberi 3 , Nathalie Villeneuve 4 , Antonio Gil-Nagel 5 , Rocio Sanchez-Carpintero 6 , Ulrich Stephani 7 , Linda Laux 8 , Elaine Wirrell 9 , Kelly Knupp 10 , Catherine Chiron 11 , Gail Farfel 2 , Bradley S Galer 2 , Glenn Morrison 2 , Michael Lock 2 , Anupam Agarwal 2 , Stéphane Auvin 12 ,
Affiliation
|
Importance
Fenfluramine treatment may reduce monthly convulsive seizure frequency in patients with Dravet syndrome who have poor seizure control with their current stiripentol-containing antiepileptic drug regimens.
Objective
To determine whether fenfluramine reduced monthly convulsive seizure frequency relative to placebo in patients with Dravet syndrome who were taking stiripentol-inclusive regimens.
Design, Setting, and Participants
This double-blind, placebo-controlled, parallel-group randomized clinical trial was conducted in multiple centers. Eligible patients were children aged 2 to 18 years with a confirmed clinical diagnosis of Dravet syndrome who were receiving stable, stiripentol-inclusive antiepileptic drug regimens.
Interventions
Patients with 6 or more convulsive seizures during the 6-week baseline period were randomly assigned to receive fenfluramine, 0.4 mg/kg/d (maximum, 17 mg/d), or a placebo. After titration (3 weeks), patients' assigned dosages were maintained for 12 additional weeks. Caregivers recorded seizures via a daily electronic diary.
Main Outcomes and Measures
The primary efficacy end point was the change in mean monthly convulsive seizure frequency between fenfluramine and placebo during the combined titration and maintenance periods relative to baseline.
Results
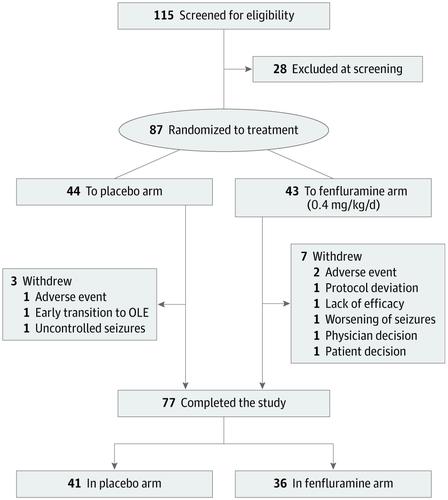
A total of 115 eligible patients were identified; of these, 87 patients (mean [SD], age 9.1 [4.8] years; 50 male patients [57%]; mean baseline frequency of seizures, approximately 25 convulsive seizures per month) were enrolled and randomized to fenfluramine, 0.4 mg/kg/d (n = 43) or placebo (n = 44). Patients treated with fenfluramine achieved a 54.0% (95% CI, 35.6%-67.2%; P < .001) greater reduction in mean monthly convulsive seizure frequency than those receiving the placebo. With fenfluramine, 54% of patients demonstrated a clinically meaningful (≥50%) reduction in monthly convulsive seizure frequency vs 5% with placebo (P < .001). The median (range) longest seizure-free interval was 22 (3.0-105.0) days with fenfluramine and 13 (1.0-40.0) days with placebo (P = .004). The most common adverse events were decreased appetite (19 patients taking fenfluramine [44%] vs 5 taking placebo [11%]), fatigue (11 [26%] vs 2 [5%]), diarrhea (10 [23%] vs 3 [7%]), and pyrexia (11 [26%] vs 4 [9%]). Cardiac monitoring demonstrated no clinical or echocardiographic evidence of valvular heart disease or pulmonary arterial hypertension.
Conclusions and Relevance
Fenfluramine demonstrated significant improvements in monthly convulsive seizure frequency in patients with Dravet syndrome whose conditions were insufficiently controlled with stiripentol-inclusive antiepileptic drug regimens. Fenfluramine was generally well tolerated. Fenfluramine may represent a new treatment option for Dravet syndrome.
Trial Registration
ClinicalTrials.gov identifier: NCT02926898.
中文翻译:

芬氟拉明用于接受Stiripentol纳入方案的Dravet综合征患者的抗治疗性癫痫发作:一项随机临床试验。
重要的是,芬氟拉明治疗可能会降低目前使用含瑞替芬太的抗癫痫药治疗癫痫发作控制不佳的Dravet综合征患者的每月惊厥发作频率。目的探讨芬氟拉明相对于安慰剂在采用甲拌异丙酚方案的Dravet综合征患者中是否降低了每月的惊厥发作频率。设计,背景和参与者该双盲,安慰剂对照,平行组随机临床试验在多个中心进行。符合条件的患者为2到18岁的儿童,他们被确诊为Dravet综合征,并接受了稳定的,包含瑞替普尔的抗癫痫药治疗。干预在6周的基线期中有6次或以上惊厥发作的患者被随机分配接受芬氟拉明,0.4 mg / kg / d(最大17 mg / d)或安慰剂治疗。滴定后(3周),患者分配的剂量再维持12周。护理人员通过每日电子日记记录癫痫发作。主要结果和措施主要疗效终点是在联合滴定和维持期间相对于基线,芬氟拉明和安慰剂之间平均每月惊厥发作频率的变化。结果共鉴定出115名符合条件的患者。在这些患者中,纳入了87例患者(平均[SD],年龄9.1 [4.8]岁; 50例男性患者[57%];平均癫痫发作的基线频率,大约每月25次惊厥性癫痫发作),并随机分配给芬氟拉明,0。4 mg / kg / d(n = 43)或安慰剂(n = 44)。与接受安慰剂的患者相比,用芬氟拉明治疗的患者平均每月惊厥发作频率降低了54.0%(95%CI,35.6%-67.2%; P <.001)。服用芬氟拉明后,有54%的患者表现出每月抽搐发作频率的临床意义(≥50%)降低,而安慰剂组则为5%(P <.001)。芬氟拉明最长无癫痫发作间隔的中位数(范围)为22(3.0-105.0)天,安慰剂为13(1.0-40.0)天(P = .004)。最常见的不良事件是食欲下降(19例服用芬氟拉明[44%] vs 5服用安慰剂[11%]),疲劳(11例[26%] vs 2 [5%]),腹泻(10例[23%] vs 3 [7%])和发热(11 [26%]对4 [9%])。心脏监护显示没有瓣膜性心脏病或肺动脉高压的临床或超声心动图证据。结论和相关性芬氟拉明显示,对于使用Dreptt综合征的患者(包括使用替替多酚的抗癫痫药不能充分控制病情),每月惊厥性癫痫发作频率有显着改善。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。
更新日期:2020-03-09
中文翻译:

芬氟拉明用于接受Stiripentol纳入方案的Dravet综合征患者的抗治疗性癫痫发作:一项随机临床试验。
重要的是,芬氟拉明治疗可能会降低目前使用含瑞替芬太的抗癫痫药治疗癫痫发作控制不佳的Dravet综合征患者的每月惊厥发作频率。目的探讨芬氟拉明相对于安慰剂在采用甲拌异丙酚方案的Dravet综合征患者中是否降低了每月的惊厥发作频率。设计,背景和参与者该双盲,安慰剂对照,平行组随机临床试验在多个中心进行。符合条件的患者为2到18岁的儿童,他们被确诊为Dravet综合征,并接受了稳定的,包含瑞替普尔的抗癫痫药治疗。干预在6周的基线期中有6次或以上惊厥发作的患者被随机分配接受芬氟拉明,0.4 mg / kg / d(最大17 mg / d)或安慰剂治疗。滴定后(3周),患者分配的剂量再维持12周。护理人员通过每日电子日记记录癫痫发作。主要结果和措施主要疗效终点是在联合滴定和维持期间相对于基线,芬氟拉明和安慰剂之间平均每月惊厥发作频率的变化。结果共鉴定出115名符合条件的患者。在这些患者中,纳入了87例患者(平均[SD],年龄9.1 [4.8]岁; 50例男性患者[57%];平均癫痫发作的基线频率,大约每月25次惊厥性癫痫发作),并随机分配给芬氟拉明,0。4 mg / kg / d(n = 43)或安慰剂(n = 44)。与接受安慰剂的患者相比,用芬氟拉明治疗的患者平均每月惊厥发作频率降低了54.0%(95%CI,35.6%-67.2%; P <.001)。服用芬氟拉明后,有54%的患者表现出每月抽搐发作频率的临床意义(≥50%)降低,而安慰剂组则为5%(P <.001)。芬氟拉明最长无癫痫发作间隔的中位数(范围)为22(3.0-105.0)天,安慰剂为13(1.0-40.0)天(P = .004)。最常见的不良事件是食欲下降(19例服用芬氟拉明[44%] vs 5服用安慰剂[11%]),疲劳(11例[26%] vs 2 [5%]),腹泻(10例[23%] vs 3 [7%])和发热(11 [26%]对4 [9%])。心脏监护显示没有瓣膜性心脏病或肺动脉高压的临床或超声心动图证据。结论和相关性芬氟拉明显示,对于使用Dreptt综合征的患者(包括使用替替多酚的抗癫痫药不能充分控制病情),每月惊厥性癫痫发作频率有显着改善。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。芬氟拉明通常耐受良好。芬氟拉明可能代表Dravet综合征的新治疗选择。试用注册ClinicalTrials.gov标识符:NCT02926898。


























 京公网安备 11010802027423号
京公网安备 11010802027423号