当前位置:
X-MOL 学术
›
ACS Earth Space Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Unraveling the Tropospheric Microhydration Processes of Iodous Acid HOIO
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2019-12-16 , DOI: 10.1021/acsearthspacechem.9b00257 Sonia Taamalli 1, 2 , Dorra Khiri 1, 2 , Siba Suliman 3 , Sarah Khanniche 1, 2 , Ivan Černušák 3 , Laurent Cantrel 2, 4 , Marc Ribaucour 1, 2 , Florent Louis 1, 2
ACS Earth and Space Chemistry ( IF 3.4 ) Pub Date : 2019-12-16 , DOI: 10.1021/acsearthspacechem.9b00257 Sonia Taamalli 1, 2 , Dorra Khiri 1, 2 , Siba Suliman 3 , Sarah Khanniche 1, 2 , Ivan Černušák 3 , Laurent Cantrel 2, 4 , Marc Ribaucour 1, 2 , Florent Louis 1, 2
Affiliation
|
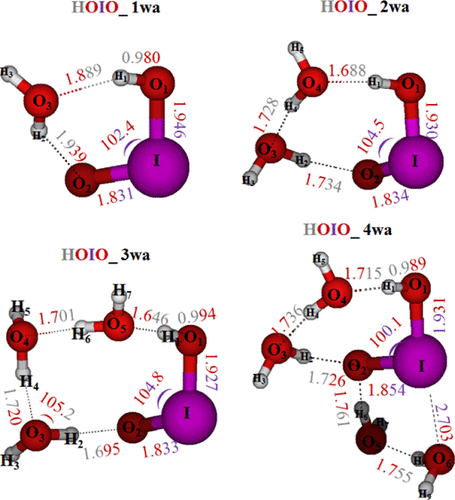
The microhydration of iodous acid has been theoretically studied using the ωB97X-D/aug-cc-pVTZ level of theory. Two hydration processes have been examined considering the addition of either successive water molecules or water clusters with a number of water molecules from 1 to 4. The singlet potential energy surface exploration revealed four monohydrates, seven dihydrates, nine trihydrates, and ten tetrahydrates. The thermodynamic properties of the hydration reactions have been calculated at different levels of theory including density functional theory and coupled cluster calculations DK-CCSD(T) with the ANO-RCC-VQZP basis sets. Standard reaction enthalpy and standard Gibbs free reaction energy were computed. The temperature dependence of ΔrG°(T) was evaluated for all studied aggregates over the temperature range of 200–400 K. The results showed that the hydrated forms of HOIO can exist in tropospheric conditions. Atmospheric implications in terms of assessing the molecular concentrations of HOIO and water aggregates are discussed.
中文翻译:

揭示碘酸HOIO的对流层微水化过程
使用ωB97X-D/ aug-cc-pVTZ理论值对碘的微水化进行了理论研究。考虑了两个水合过程,考虑了添加连续的水分子或具有1至4的许多水分子的水团簇。单重态势能表面探测显示四个一水合物,七个二水合物,九个三水合物和十个四水合物。水化反应的热力学性质已在不同的理论水平上进行了计算,包括密度泛函理论和具有ANO-RCC-VQZP基集的耦合簇计算DK-CCSD(T)。计算标准反应焓和标准吉布斯自由反应能。温度依赖性Δr G °(T)在200–400 K的温度范围内对所有研究的聚集体进行了评估。结果表明,HOIO的水合形式可以存在于对流层条件下。讨论了评估HOIO和水聚集体的分子浓度对大气的影响。
更新日期:2019-12-17
中文翻译:

揭示碘酸HOIO的对流层微水化过程
使用ωB97X-D/ aug-cc-pVTZ理论值对碘的微水化进行了理论研究。考虑了两个水合过程,考虑了添加连续的水分子或具有1至4的许多水分子的水团簇。单重态势能表面探测显示四个一水合物,七个二水合物,九个三水合物和十个四水合物。水化反应的热力学性质已在不同的理论水平上进行了计算,包括密度泛函理论和具有ANO-RCC-VQZP基集的耦合簇计算DK-CCSD(T)。计算标准反应焓和标准吉布斯自由反应能。温度依赖性Δr G °(T)在200–400 K的温度范围内对所有研究的聚集体进行了评估。结果表明,HOIO的水合形式可以存在于对流层条件下。讨论了评估HOIO和水聚集体的分子浓度对大气的影响。


























 京公网安备 11010802027423号
京公网安备 11010802027423号