当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Expanding the Frontiers of Higher-Order Cycloadditions.
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.accounts.9b00498 David McLeod 1 , Mathias Kirk Thøgersen 1 , Nicolaj Inunnguaq Jessen 1 , Karl Anker Jørgensen 1 , Cooper S Jamieson 2 , Xiao-Song Xue 2 , K N Houk 2 , Fang Liu 3 , Roald Hoffmann 4
Accounts of Chemical Research ( IF 18.3 ) Pub Date : 2019-12-02 , DOI: 10.1021/acs.accounts.9b00498 David McLeod 1 , Mathias Kirk Thøgersen 1 , Nicolaj Inunnguaq Jessen 1 , Karl Anker Jørgensen 1 , Cooper S Jamieson 2 , Xiao-Song Xue 2 , K N Houk 2 , Fang Liu 3 , Roald Hoffmann 4
Affiliation
|
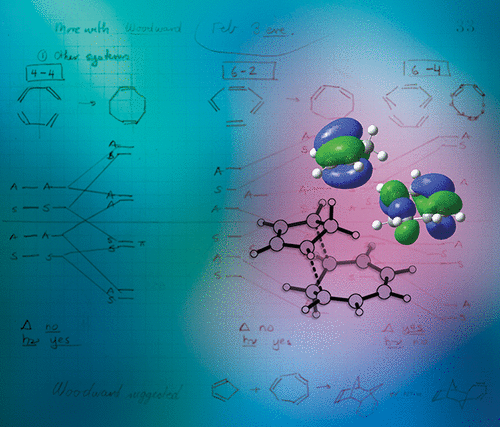
The concept of pericyclic reactions and the explanation of their specificity through orbital symmetries introduced a new way of understanding reactions and looking for new ones. One of the 1965 Woodward-Hoffmann communications described "the (as yet unobserved) symmetry-allowed 6 + 4 combination", the prediction of a new field of "higher-order" cycloadditions, involving more than six electrons. Later these authors predicted exo-stereoselectivity for the [6 + 4]-cycloaddition. Chemists rushed to test this prediction (for the most part successfully). For more than half a century, chemists have hunted for additional higher-order cycloadditions. The application of catalysis within organic chemistry allows the accomplishment of previously unattainable reactions, including higher-order cycloadditions. The many examples of [8 + 2], [6 + 4], and cycloadditions of even higher electron-counts discovered since the Woodward-Hoffmann rules were introduced illustrate the difficulty in predicting which of these transformations will occur when two highly unsaturated molecules react. Periselectivity has been a challenge, and the development of enantioselective variants has been elusive. While progress was made, the rise of organocatalysis in asymmetric synthesis has led to a surge of interest in stereoselective versions of higher-order cycloadditions. Through organocatalytic activation of conjugated cyclic polyenes and heteroaromatic compounds, asymmetric [8 + 2]-, [6 + 4]-, and [10 + 4]-cycloadditions have been realized by our groups. In this century, [6 + 4]-cycloadditions have been found also to occur in enzyme-catalyzed reactions for the biosynthesis of spinosyn A, heronamide, and streptoseomycin natural products. A whole new class of enzymes, the pericyclases that catalyze pericyclic reactions, has been discovered. A remarkable aspect of these recent developments is the cross-disciplinary research involved: from organic synthesis to computational studies integrated with experimental studies of reaction mechanisms, intermediates, and dynamics, to understanding mechanisms of enzyme catalysis and engineering of enzymes. This Account describes how our groups have been involved in the expansion of the higher-order cycloaddition frontiers. We describe both the history and recent progress in higher-order cycloadditions, and how these advances have been made by our collaborative experimental and computational studies. Progress in asymmetric organocatalysis, incorporating enantioselective higher-order cycloadditions in organic synthesis, and the stereoselective synthesis of important scaffolds will be highlighted. Experimental progress and computational modeling with density functional theory (DFT) has identified ambimodal cycloaddition pathways and led to the realization that multiple products of pericyclic reactions are linked by common transition states. Molecular dynamic simulations have provided fundamental understanding of factors controlling periselectivity and have led to discoveries of a group of enzymes, the pericyclases, which catalyze pericyclic reactions such as [6 + 4]-cycloadditions.
中文翻译:

扩展高阶环加成的前沿。
周环反应的概念和通过轨道对称性对其特异性的解释引入了一种理解反应和寻找新反应的新方法。1965年的伍德沃德-霍夫曼(Woodward-Hoffmann)通讯中的一项描述了“(至今尚未观察到)对称性允许的6 + 4组合”,这是“高阶”环加成新领域的预言,涉及超过六个电子。后来,这些作者预测了[6 + 4]-环加成反应的立体立体选择性。化学家急于测试这一预测(大部分成功)。半个多世纪以来,化学家一直在寻找其他高阶环加成物。催化在有机化学中的应用可以完成以前无法实现的反应,包括高阶环加成反应。[8 + 2]的许多示例,[6 + 4],以及自引入伍德沃德-霍夫曼规则以来发现的更高电子数的环加成反应,说明了很难预测当两个高度不饱和的分子发生反应时将发生哪些转变。全选择性一直是一个挑战,对映选择性变体的开发一直难以捉摸。尽管取得了进展,但不对称合成中有机催化的兴起引起了对高阶环加成的立体选择形式的兴趣激增。通过共轭环状多烯和杂芳族化合物的有机催化活化,我们的小组已经实现了不对称的[8 + 2]-,[6 + 4]-和[10 + 4]-环加成。在本世纪,还发现了[6 + 4]-环加成反应也发生在酶催化反应中,用于生物合成多杀菌素A,heronamide,和链霉素天然产物。已经发现了一种全新的酶,即催化周环反应的周环酶。这些最新进展的一个显着方面是涉及的跨学科研究:从有机合成到将反应机理,中间体和动力学的实验研究结合在一起的计算研究,再到对酶催化机理和酶工程学的理解。此帐户描述了我们的团队如何参与高阶环加成前沿的扩展。我们描述了高阶环加成反应的历史和最新进展,以及我们的合作实验和计算研究如何取得这些进展。不对称有机催化的进展,在有机合成中结合对映选择性的高阶环加成反应,以及重要骨架的立体选择性合成将得到重点介绍。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。
更新日期:2019-12-02
中文翻译:

扩展高阶环加成的前沿。
周环反应的概念和通过轨道对称性对其特异性的解释引入了一种理解反应和寻找新反应的新方法。1965年的伍德沃德-霍夫曼(Woodward-Hoffmann)通讯中的一项描述了“(至今尚未观察到)对称性允许的6 + 4组合”,这是“高阶”环加成新领域的预言,涉及超过六个电子。后来,这些作者预测了[6 + 4]-环加成反应的立体立体选择性。化学家急于测试这一预测(大部分成功)。半个多世纪以来,化学家一直在寻找其他高阶环加成物。催化在有机化学中的应用可以完成以前无法实现的反应,包括高阶环加成反应。[8 + 2]的许多示例,[6 + 4],以及自引入伍德沃德-霍夫曼规则以来发现的更高电子数的环加成反应,说明了很难预测当两个高度不饱和的分子发生反应时将发生哪些转变。全选择性一直是一个挑战,对映选择性变体的开发一直难以捉摸。尽管取得了进展,但不对称合成中有机催化的兴起引起了对高阶环加成的立体选择形式的兴趣激增。通过共轭环状多烯和杂芳族化合物的有机催化活化,我们的小组已经实现了不对称的[8 + 2]-,[6 + 4]-和[10 + 4]-环加成。在本世纪,还发现了[6 + 4]-环加成反应也发生在酶催化反应中,用于生物合成多杀菌素A,heronamide,和链霉素天然产物。已经发现了一种全新的酶,即催化周环反应的周环酶。这些最新进展的一个显着方面是涉及的跨学科研究:从有机合成到将反应机理,中间体和动力学的实验研究结合在一起的计算研究,再到对酶催化机理和酶工程学的理解。此帐户描述了我们的团队如何参与高阶环加成前沿的扩展。我们描述了高阶环加成反应的历史和最新进展,以及我们的合作实验和计算研究如何取得这些进展。不对称有机催化的进展,在有机合成中结合对映选择性的高阶环加成反应,以及重要骨架的立体选择性合成将得到重点介绍。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。实验进展和使用密度泛函理论(DFT)的计算模型已经确定了双峰环加成途径,并导致认识到周环反应的多种产物通过共同的过渡态联系在一起。分子动力学模拟已经提供了对控制周选择性的因素的基本理解,并导致发现了一组酶,即周环化酶,这些酶催化周向反应,例如[6 + 4]-环加成反应。



























 京公网安备 11010802027423号
京公网安备 11010802027423号